Korean J Radiol.
2018 Jun;19(3):470-480. 10.3348/kjr.2018.19.3.470.
Utility of Quantitative Parameters from Single-Photon Emission Computed Tomography/Computed Tomography in Patients with Destructive Thyroiditis
- Affiliations
-
- 1Department of Nuclear Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam 13620, Korea. wwlee@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam 13620, Korea.
- 3Department of Nuclear Medicine, Konkuk University Medical Center, Seoul 05030, Korea.
- 4Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul 03080, Korea.
- KMID: 2410818
- DOI: http://doi.org/10.3348/kjr.2018.19.3.470
Abstract
OBJECTIVE
Quantitative parameters from Tc-99m pertechnetate single-photon emission computed tomography/computed tomography (SPECT/CT) are emerging as novel diagnostic markers for functional thyroid diseases. We intended to assess the utility of SPECT/CT parameters in patients with destructive thyroiditis.
MATERIALS AND METHODS
Thirty-five destructive thyroiditis patients (7 males and 28 females; mean age, 47.3 ± 13.0 years) and 20 euthyroid patients (6 males and 14 females; mean age, 45.0 ± 14.8 years) who underwent Tc-99m pertechnetate quantitative SPECT/CT were retrospectively enrolled. Quantitative parameters from the SPECT/CT (%uptake, standardized uptake value [SUV], thyroid volume, and functional thyroid mass [SUVmean × thyroid volume]) and thyroid hormone levels were investigated to assess correlations and predict the prognosis for destructive thyroiditis. The occurrence of hypothyroidism was the outcome for prognosis.
RESULTS
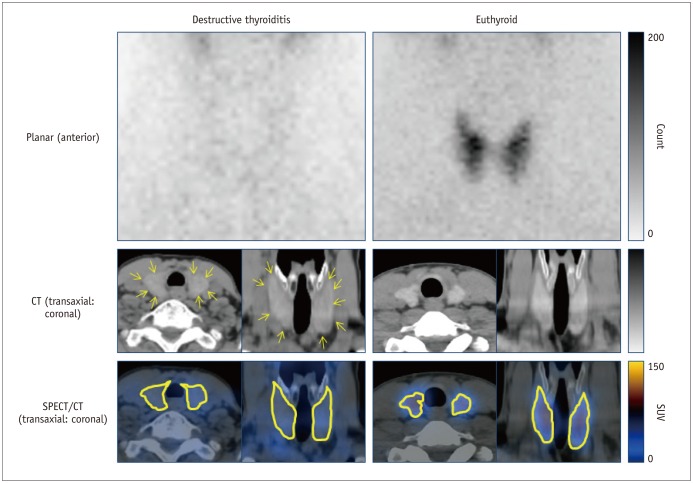
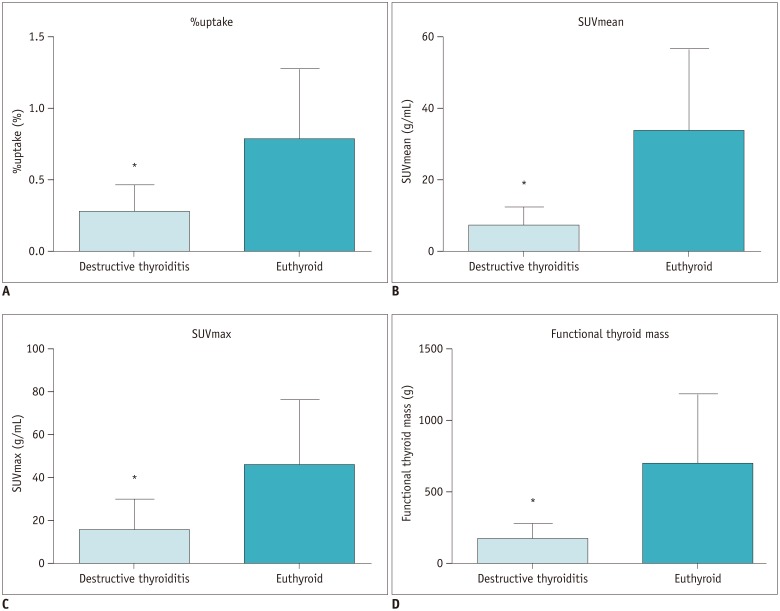
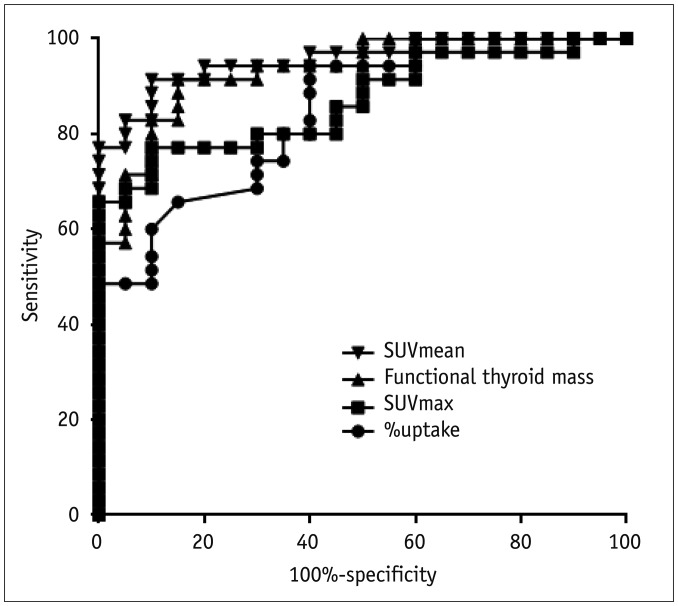
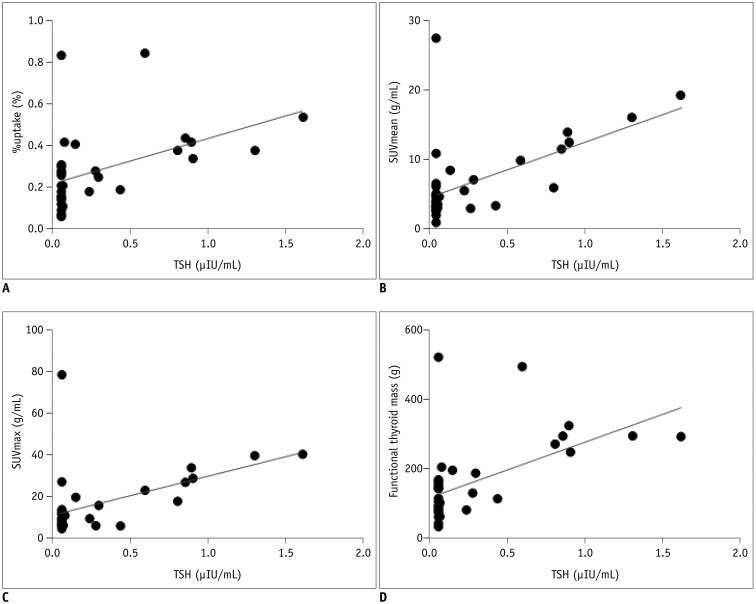
All the SPECT/CT quantitative parameters were significantly lower in the 35 destructive thyroiditis patients compared to the 20 euthyroid patients using the same SPECT/CT scanner and protocol (p < 0.001 for all parameters). T3 and free T4 did not correlate with any SPECT/CT parameters, but thyroid-stimulating hormone (TSH) significantly correlated with %uptake (p = 0.004), SUVmean (p < 0.001), SUVmax (p = 0.002), and functional thyroid mass (p < 0.001). Of the 35 destructive thyroiditis patients, 16 progressed to hypothyroidism. On univariate and multivariate analyses, only T3 levels were associated with the later occurrence of hypothyroidism (p = 0.002, exp(β) = 1.022, 95% confidence interval: 1.008 - 1.035).
CONCLUSION
Novel quantitative SPECT/CT parameters could discriminate patients with destructive thyroiditis from euthyroid patients, suggesting the robustness of the quantitative SPECT/CT approach. However, disease progression of destructive thyroiditis could not be predicted using the parameters, as these only correlated with TSH, but not with T3, the sole predictor of the later occurrence of hypothyroidism.
Keyword
MeSH Terms
Figure
Reference
-
1. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003; 348:2646–2655. PMID: 12826640.
Article2. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016; 26:1343–1421. PMID: 27521067.
Article3. Volpé R, Row VV, Ezrin C. Circulating viral and thyroid antibodies in subacute thyroiditis. J Clin Endocrinol Metab. 1967; 27:1275–1284. PMID: 4292248.4. Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003; 88:2100–2105. PMID: 12727961.
Article5. Muller AF, Drexhage HA, Berghout A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev. 2001; 22:605–630. PMID: 11588143.
Article6. Mittra ES, McDougall IR. Recurrent silent thyroiditis: a report of four patients and review of the literature. Thyroid. 2007; 17:671–675. PMID: 17696838.
Article7. Slatosky J, Shipton B, Wahba H. Thyroiditis: differential diagnosis and management. Am Fam Physician. 2000; 61:1047–1052. PMID: 10706157.8. Sicilia V, Mezitis S. A case of acute suppurative thyroiditis complicated by thyrotoxicosis. J Endocrinol Invest. 2006; 29:997–1000. PMID: 17259797.
Article9. Shigemasa C, Kouchi T, Taniguchi S, Mitani Y, Ueta Y, Yoshida A, et al. Autoimmune thyroiditis with transient thyrotoxicosis: comparison between painful thyroiditis and painless thyroiditis. Horm Res. 1991; 36:9–15. PMID: 1814808.
Article10. Atkins HL, Fleay RF. Data blending with 99mTc in evaluating thyroid anatomy by scintillation scanning. J Nucl Med. 1968; 9:66–73. PMID: 5635237.11. Meller J, Becker W. The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound. Eur J Nucl Med Mol Imaging. 2002; 29(2):S425–S438. PMID: 12192542.
Article12. Ritt P, Vija H, Hornegger J, Kuwert T. Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging. 2011; 38(1):S69–S77. PMID: 21484383.
Article13. Bailey DL, Willowson KP. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med. 2013; 54:83–89. PMID: 23283563.
Article14. Cachovan M, Vija AH, Hornegger J, Kuwert T. Quantification of 99mTc-DPD concentration in the lumbar spine with SPECT/CT. EJNMMI Res. 2013; 3:45. PMID: 23738809.
Article15. Suh MS, Lee WW, Kim YK, Yun PY, Kim SE. Maximum standardized uptake value of (99m)Tc hydroxymethylene diphosphonate SPECT/CT for the evaluation of temporomandibular joint disorder. Radiology. 2016; 280:890–896. PMID: 27035060.16. Lee H, Kim JH, Kang YK, Moon JH, So Y, Lee WW. Quantitative single-photon emission computed tomography/computed tomography for technetium pertechnetate thyroid uptake measurement. Medicine (Baltimore). 2016; 95:e4170. PMID: 27399139.
Article17. Kim HJ, Bang JI, Kim JY, Moon JH, So Y, Lee WW. Novel application of quantitative single-photon emission computed tomography/computed tomography to predict early response to methimazole in Graves' disease. Korean J Radiol. 2017; 18:543–550. PMID: 28458607.
Article18. Chang KJ, Lim I, Park JY, Jo AR, Kong CB, Song WS, et al. The role of (18)F-FDG PET/CT as a prognostic factor in patients with synovial sarcoma. Nucl Med Mol Imaging. 2015; 49:33–41. PMID: 25774236.
Article19. Park S, Lee E, Rhee S, Cho J, Choi S, Lee S, et al. Correlation between semi-quantitative (18)F-FDG PET/CT parameters and Ki-67 expression in small cell lung cancer. Nucl Med Mol Imaging. 2016; 50:24–30. PMID: 26941856.
Article20. Cooper DS. Clinical practice. Subclinical hypothyroidism. N Engl J Med. 2001; 345:260–265. PMID: 11474665.21. Van Sande J, Massart C, Beauwens R, Schoutens A, Costagliola S, Dumont JE, et al. Anion selectivity by the sodium iodide symporter. Endocrinology. 2003; 144:247–252. PMID: 12488351.
Article22. Lee WW, Moon DH, Park SY, Jin J, Kim SJ, Lee H. Imaging of adenovirus-mediated expression of human sodium iodide symporter gene by 99mTcO4 scintigraphy in mice. Nucl Med Biol. 2004; 31:31–40. PMID: 14741568.23. Chung JK, Youn HW, Kang JH, Lee HY, Kang KW. Sodium iodide symporter and the radioiodine treatment of thyroid carcinoma. Nucl Med Mol Imaging. 2010; 44:4–14. PMID: 24899932.
Article24. Riedel C, Levy O, Carrasco N. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem. 2001; 276:21458–21463. PMID: 11290744.
Article25. Lee WW, Lee B, Kim SJ, Jin J, Moon DH, Lee H. Kinetics of iodide uptake and efflux in various human thyroid cancer cells by expressing sodium iodide symporter gene via a recombinant adenovirus. Oncol Rep. 2003; 10:845–849. PMID: 12792733.
Article26. Amino N, Yabu Y, Miyai K, Fujie T, Azukizawa M, Onishi T, et al. Differentiation of thyrotoxicosis induced by thyroid destruction from Graves’ disease. Lancet. 1978; 2:344–346. PMID: 79711.
Article27. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002; 23:38–89. PMID: 11844744.
Article28. Yanagisawa T, Sato K, Kato Y, Shimizu S, Takano K. Rapid differential diagnosis of Graves’ disease and painless thyroiditis using total T3/T4 ratio, TSH, and total alkaline phosphatase activity. Endocr J. 2005; 52:29–36. PMID: 15758555.
Article29. Teixeira VL, Romaldini JH, Rodrigues HF, Tanaka LM, Farah CS. Thyroid function during the spontaneous course of subacute thyroiditis. J Nucl Med. 1985; 26:457–460. PMID: 3921671.30. Nicoloff JT, Lum SM, Spencer CA, Morris R. Peripheral autoregulation of thyroxine to triiodothyronine conversion in man. Horm Metab Res Suppl. 1984; 14:74–79. PMID: 6595193.31. Carlé A, Knudsen N, Pedersen IB, Perrild H, Ovesen L, Rasmussen LB, et al. Determinants of serum T4 and T3 at the time of diagnosis in nosological types of thyrotoxicosis: a population-based study. Eur J Endocrinol. 2013; 169:537–545. PMID: 23935127.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE: Novel Application of Quantitative Single-Photon Emission Computed Tomography/Computed Tomography to Predict Early Response to Methimazole in Graves' Disease
- Nuclear Medicine Imaging in Rheumatic Diseases
- Clinical Applications of Technetium-99m Quantitative Single-Photon Emission Computed Tomography/Computed Tomography
- Diagnosis of hepatic hemangioma with 99mTc-labeled red cells and single photon emission computed tomography (SPECT)
- A Case of Orbital Cavernous Hemangioma with Multiple Intra c ranial Lesions