J Korean Soc Echocardiogr.
1996 Jul;4(1):5-12. 10.4250/jkse.1996.4.1.5.
Effects of L-Arginine on the Change of Myocardial Stunning
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Korea University, Seoul, Korea.
- KMID: 2410400
- DOI: http://doi.org/10.4250/jkse.1996.4.1.5
Abstract
- BACKGROUND
The effects of L-arginine-NO system on myocardial stunning haven't been well known. This work was designed to know whether L-arginine, physiologic NO precusor, would attenuate postischemic myocardial dysfunction or not. To investigate whether intravenous administration of L-arginine, physiological nitric oxide(NO) precursor, during reperfusion would attenuate postischemic myocardial dysfunction, 18 open-chest dogs were studied.
METHODS
In 18 pentobarbital anesthesized open-chest dogs, left circumflex coronary artery was occluded for 20 minutes and was followed by a reperfusion for 60 minutes. L-Arginine(30mg/kg)(L-arginine group, n=8) or saline(control group, n=10) was infused intravenously 1 minute before reperfusion and was followed by a continuous infusion (10mg/kg/min) for 30 minutes during reperfusion. Before coronary occlusion and 30 minutes and 60 minutes after reperfusion, coronary blood flow(CBF) and coronary vascular resistance(CVR) were measured. Myocardial segment thickening in the area of ischemia-reperfusion was measured using 2D-echocardiography. The echocardiography images were digitized and analyzed by cardiac image analyzer.
RESULTS
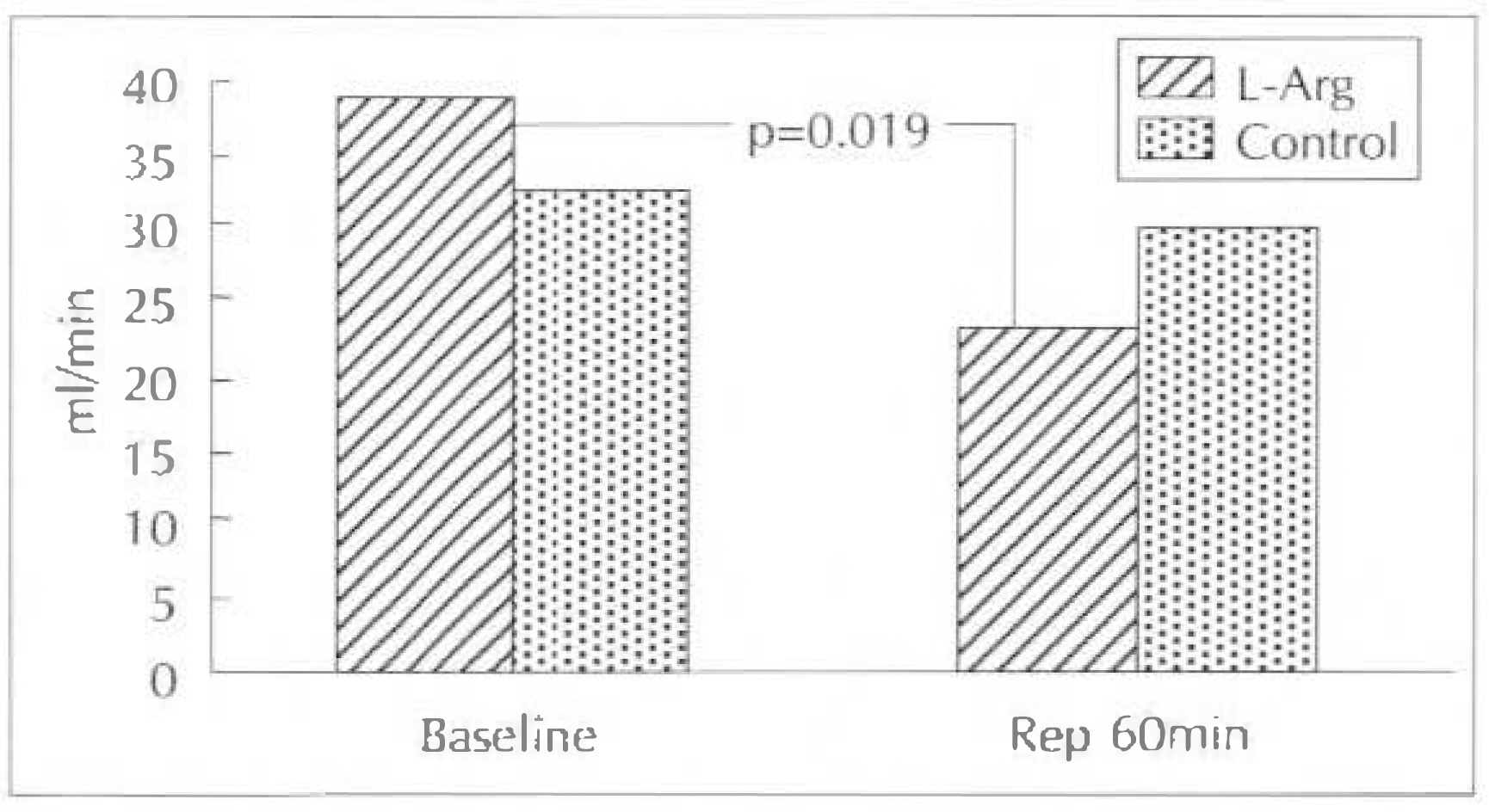
1) Percent change of CBF was decreased by 42.5% in L-arginine group but it was increased by 1.3% in control group(p=0.025) and %change of CVR was increased by 83.5% in L-arginine group vs 11% in control group after 60 minutes of reperfusion, compared with pre-occlusion baseline values(p=0.06). 2) Percent change of myocardial segment thickening was decreased both in L-arginine group(by 69.5%) and control group(by 57.6%) after reperfusion 30 minutes without statistically significance, but it was significantly decreased in L-arginine group(by 80%) compared with control group(by 55.6%) after reperfusion 60 minute(p=0.01).
CONCLUSION
The findings that the administration of L-arginine cause significant depression of post-ischemic myocardial contractile function after reperfusion 60 minutes suggests that systemic infusion of L-arginine has an unfavorable effect on myocardial stunning and low reflow phenomenon. These results suggest that L-arginine may have independent deteriorating effects on myocardial stunning after reperfusion 60 minutes.
Keyword
MeSH Terms
Figure
Reference
-
References
1). 김홍균 권년수: Nitric Oxide의 생성과 그 기능. Med Postgraduates. 21:274. 1993.2). Anggard E. Nitric oxide: Mediator, murderer, and medicine. Lancet. 343:1199. 1994.3). Bredt DS, Snyder SH. Isolation of nitric oxide synthetase. a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 87:682. 1990.
Article4). Del Mastro RF, Bjor J, Arfor KE. Increase in microvascular permeability induced by enzymatically generated free radicals. Microvasc Res. 22:239. 1981.5). Depre C, Vanoverschelde JL, Goudemann , Mottet I, Hue L. Protection aganist ischemic injury by non-vasoactive concentrations of nitric oxide synthase inhibitors in the perfused rabbit heart. Circulation. 92:1911–1918. 1995.6). Eldor A, Falcone DJ, Hajjar DP, Minick CR, Weksler BB. Recovery of postacyclin production by deendothelialized rabbit aorta: Critical role of the neointimal smooth muscle cells. J Clin Invest. 67:735. 1981.7). Forstermann U, Pollock JS, Schmidt HHHW, Heller M, Murad F. Calmodulin-dependent endolium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci USA. 88:1788. 1991.8). Green LC, Tannenbaum SR, Goldman P. Nitrate synthesis in the germfree and and conventional rat. Science. 212:56. 1981.9). Hasebe N, Shen YT, Vantner SF. Inhibition of endothelium-derived relaxing factor enhances myocardial stunning in conscious dogs. Circuklation. 88:2862. 1993.
Article10). Hiki K, Hattori R, Kawai C, Yui Y. Purification of insoluble nitric oxide synthase from rat cerebellum. J Biochem. 111:556. 1992.11). Kasuya Y, Ishikawa T, Yanagisawa M, et al. Mechanism of contraction to endothelin in scolated porcine coronary artery. Am J Physiol. 257:HI828. 1989.12). Kowski W, Luscher TF, Linder L, Buhler FR. Endothelin-1-induced vasoconstriction in man: Reversal by calcium channel blockade but not by nitrovasodilators or endothelium-derived relaxing factor. Circulation. 83:469. 1991.13). Kubes P. Nitric oxide modulates epithelial permeability in the feline small intestine. Am J Physiol. 262:G1138. 1992.
Article14). Lowenstein CJ, Dinerman JL, Synder SH. Nitric oxide: A physiologic messenger. Ann Intern Med. 120:227. 1994.
Article15). Luscher TF, Boulanger CM, Dohi Y, Yang Z. Endothelium-derived contracting factors. Hypertension. 19:117. 1992.
Article16). Marietta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 268:12231. 1993.17). Mathesis G, Sherman MP, Buckberg GD, Haybron DM, Young HH, Ignarro LJ. Role of L-ariginine-nitric oxide pathway in myocardial reoxygenation injury. Am Physiol Soci. H616:1992.18). Moncada S, Higgs A. The L-arginine-nitric oxide pathway. New Engl J Med. 2002:1993.19). Moncada S, Palmer RMJ, Higgs EA. Biosynthesis of nitric oxide for L-arginine: A pathway for the regulation of cell function and communication. Biochem Pharmacol. 38:1709. 1989.20). Moncada S, Palmer rmj, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 43:109. 1991.21). Morishima N, Matsuura H, Shiode N, Shinohara K, Karakawa S, Yamagata T, Kajiyama G. L-arginine deteriorates contractile function in repetitively occlude-reperfused myocardium. J Am Coll Cardiol. 263A:1994.22). Naseem SA, Kontos MC, Rao PS, Jesse RL, Hess ML, Kukreja RC. Sustained inhibition of nitric oxide by N-nitro-L-arginine improves myocardial faction following ischemia/reperfusion in isolated perfused rat heart. J Mol Cell Cardiol. 27:419–426. 1995.23). Oliver JA. Endothelium-derived relaxing factor contributes to the regulation of endothelial permeability. J Cell Physiol. 151:506. 1992.
Article24). Peach MJ, Loeb AL, Singer HA, Saye J. Endothelium-derived vascular relaxing factor. Hypertension. 7:(. (Suppl I):):. 1–94. 1985.
Article25). Pollock JS, Forstermann U, Mitchell JA, et al. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA. 88:10480. 1991.
Article26). Radomski MW, Palmer RMJ, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 2:1057. 1987.
Article27). Schmidt HHHW, Pollock JS, Nakane M, Gorsky LD, Forstermann U, Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad SCi USA. 88:365. 1991.
Article28). Schmidt HHW, Warner TD, Ishii K, Sheng H, Murad F. Insulin secretion from pancreatic B cells caused by I-arginine-derived nitrogen oxides. Science. 255:721–723. 1992.29). Sobey CG, GJ Dusting, HJ Grossman, Woodman OL. Impaired vasodilatation of epicardial coronary arteries and resistance vassels following myocardial ischemia and reperfusion in anesthetized dogs. Cor Art Dis. 1:363. 1990.30). Tsao PS, N Aoki, DJ Lefer, G Johnson III, Lefer AM. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation. 82:1402. 1990.
Article31). VanBenthuysen KM, IF McMurtry, Horwitz LD. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J Clin Invest. 79:265. 1987.
Article32). Wright CL, Rees DD, Moncada S. Protective and pathophysiological role of nitric oxide in endotoxin shock. Cardiovasc Res. 26:48–57. 1992.33). Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 332:411. 1988.
Article34). Yao SK, Ober JC, Krishnaswami A, Ferguson JJ, Anderson HV, Golino P, Buja LM, Willerson JT. Endogenous nitric oxide protects against platelet aggregation and cyclic flow variations in stenosed and endothelium-injured arteries. Circulation. 86:1302. 1992.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of L-Arginine on Post-Ischemic Myocardial and Vascular Stunning in Open-Chest Dogs
- A Case of Myocardial Stunning in Hyperthyroidism
- Effects of Ischemic preconditioning on the Post-ischemic Myocardial Dysfunction and Coronary Flow in the Isolated Rat Hearts
- Effects of Myocardial Stunning on Remote Coronary Flow Reserve
- The clinical significance of the differernce in left ventricular ejection fraction between rest and stress on gated myocardial perfusion SPECT