Korean J Physiol Pharmacol.
2018 May;22(3):291-300. 10.4196/kjpp.2018.22.3.291.
Establishment and evaluation of the VX2 orthotopic lung cancer rabbit model: a ultra-minimal invasive percutaneous puncture inoculation method
- Affiliations
-
- 1Department of Pharmacy, Chongqing Engineering Research Center of Pharmaceutical Sciences, Chongqing Medical and Pharmaceutical College, Chongqing 401331, China.
- 2Pharmacy College, Chongqing Medical University, Chongqing 400016, China. yuyu3519@163.com
- 3Department of Pharmacy, Chongqing General Hospital, Chongqing 400014, China.
- 4Radiology Department, Chongqing General Hospital, Chongqing 400014, China.
- KMID: 2410093
- DOI: http://doi.org/10.4196/kjpp.2018.22.3.291
Abstract
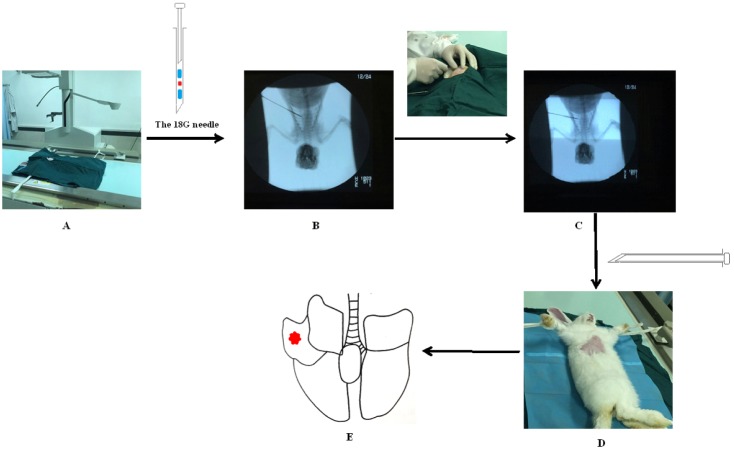
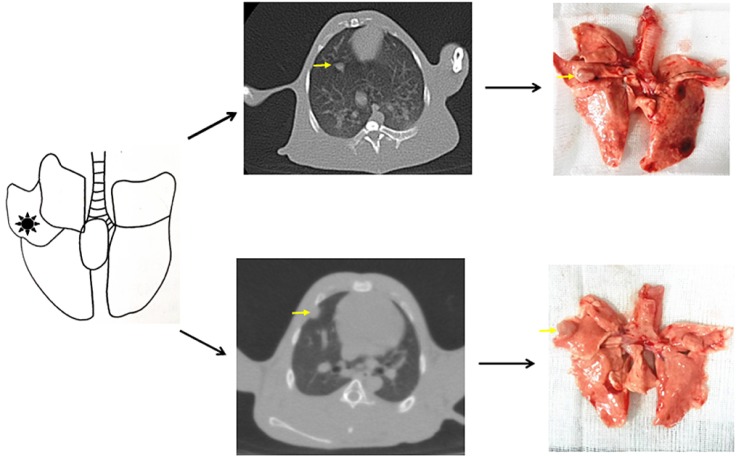
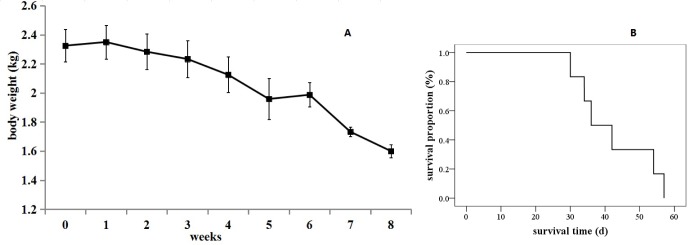
- The purpose of the present work is to establish an ultra-minimal invasive percutaneous puncture inoculation method for a VX2 orthotopic lung cancer rabbit model with fewer technical difficulties, lower mortality of rabbits, a higher success rate and a shorter operation time, to evaluate the growth, metastasis and apoptosis of tumor by CT scans, necropsy, histological examination, flow cytometry and immunohistochemistry. The average inoculation time was 10-15 min per rabbit. The tumor-bearing rate was 100%. More than 90% of the tumor-bearing rabbits showed local solitary tumor with 2-10 mm diameters after two weeks post-inoculation, and the rate of chest seeding was only 8.3% (2/24). The tumors diameters increased to 4-16 mm, and irregularly short thorns were observed 3 weeks after inoculation. Five weeks post-inoculation, the liquefaction necrosis and a cavity developed, and the size of tumor grew further. Before natural death, the CT images showed that the tumors spread to the chest. The flow cytometry and immunohistochemistry indicated that there was less apoptosis in VX2 orthotopic lung cancer rabbit model compared to chemotherapy drug treatment group. Minimal invasive percutaneous puncture inoculation is an easy, fast and accurate method to establish the VX2 orthotopic lung cancer rabbit model, an ideal in situ tumor model similar to human malignant tumor growth.
Keyword
MeSH Terms
Figure
Reference
-
1. Stewart BW, Wild CP. World Cancer Report 2014. Lyon: WHO International agency for research on Cancer;2014.2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30. PMID: 26742998.
Article3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–132. PMID: 26808342.
Article4. Zhao L, Wei Y, Li W, Liu Y, Wang Y, Zhong X, Yu Y. Solid dispersion and effervescent techniques used to prepare docetaxel liposomes for lung-targeted delivery system: in vitro and in vivo evaluation. J Drug Target. 2011; 19:171–178. PMID: 20429774.5. Razi SS, Rehmani S, Li X, Park K, Schwartz GS, Latif MJ, Bhora FY. Antitumor activity of paclitaxel is significantly enhanced by a novel proapoptotic agent in non-small cell lung cancer. J Surg Res. 2015; 194:622–630. PMID: 25498514.
Article6. Alibolandi M, Ramezani M, Abnous K, Sadeghi F, Atyabi F, Asouri M, Ahmadi AA, Hadizadeh F. In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J Control Release. 2015; 209:88–100. PMID: 25912964.
Article7. Cho WY, Hong SH, Singh B, Islam MA, Lee S, Lee AY, Gankhuyag N, Kim JE, Yu KN, Kim KH, Park YC, Cho CS, Cho MH. Suppression of tumor growth in lung cancer xenograft model mice by poly(sorbitol-co-PEI)-mediated delivery of osteopontin siRNA. Eur J Pharm Biopharm. 2015; 94:450–462. PMID: 26141346.
Article8. Chi L, Na MH, Jung HK, Vadevoo SM, Kim CW, Padmanaban G, Park TI, Park JY, Hwang I, Park KU, Liang F, Lu M, Park J, Kim IS, Lee BH. Enhanced delivery of liposomes to lung tumor through targeting interleukin-4 receptor on both tumor cells and tumor endothelial cells. J Control Release. 2015; 209:327–336. PMID: 25979323.
Article9. Liu X, Liu J, Guan Y, Li H, Huang L, Tang H, He J. Establishment of an orthotopic lung cancer model in nude mice and its evaluation by spiral CT. J Thorac Dis. 2012; 4:141–145. PMID: 22833819.10. Isobe T, Onn A, Morgensztern D, Jacoby JJ, Wu W, Shintani T, Itasaka S, Shibuya K, Koo PJ, O'Reilly MS, Herbst RS. Evaluation of novel orthotopic nude mouse models for human small-cell lung cancer. J Thorac Oncol. 2013; 8:140–146. PMID: 23328546.
Article11. Iochmann S, Lerondel S, Bléchet C, Lavergne M, Pesnel S, Sobilo J, Heuzé-Vourc'h N, Le Pape A, Reverdiau P. Monitoring of tumour progression using bioluminescence imaging and computed tomography scanning in a nude mouse orthotopic model of human small cell lung cancer. Lung Cancer. 2012; 77:70–76. PMID: 22321610.
Article12. Shope RE, Hurst EW. Infectious papillomatosis of rabbits: with a note on the histopathology. J Exp Med. 1933; 58:607–624. PMID: 19870219.13. Rous P, Beard JW. The progression to carcinoma of virus-induced rabbit papillomas (Shope). J Exp Med. 1935; 62:523–548. PMID: 19870432.
Article14. Qin H, Zhang MR, Xie L, Hou Y, Hua Z, Hu M, Wang Z, Wang F. PET imaging of apoptosis in tumor-bearing mice and rabbits after paclitaxel treatment with (18)F(-)Labeled recombinant human His10-annexin V. Am J Nucl Med Mol Imaging. 2014; 5:27–37. PMID: 25625024.15. Okuma T, Matsuoka T, Okamura T, Wada Y, Yamamoto A, Oyama Y, Koyama K, Nakamura K, Watanabe Y, Inoue Y. 18F-FDG smallanimal PET for monitoring the therapeutic effect of CT-guided radiofrequency ablation on implanted VX2 lung tumors in rabbits. J Nucl Med. 2006; 47:1351–1358. PMID: 16883016.16. Zhang Q, Shi B, Liu Z, Zhang M, Zhang W. Preliminary study of CT in combination with MRI perfusion imaging to assess hemodynamic changes during angiogenesis in a rabbit model of lung cancer. Onco Targets Ther. 2013; 6:685–692. PMID: 23836981.
Article17. Tu M, Xu L, Wei X, Miao Y. How to establish a solitary and localized VX2 lung cancer rabbit model? A simple and effective intrapulmonary tumor implantation technique. J Surg Res. 2009; 154:284–292. PMID: 18755479.
Article18. Xu Yp, Yang M, Pan Dh, Wang Lz, Liu L, Huang P, Shao G. Bioevaluation study of 32P-CP-PLLA particle brachytherapy in a rabbit VX2 lung tumor model. Appl Radiat Isot. 2012; 70:583–588. PMID: 22245365.
Article19. Lin LM, Chen YK, Chen CH, Chen YW, Huang AH, Wang WC. VX2-induced rabbit buccal carcinoma: a potential cancer model for human buccal mucosa squamous cell carcinoma. Oral Oncol. 2009; 45:e196–e203. PMID: 19666238.
Article20. Chen J, White SB, Harris KR, Li W, Yap JW, Kim DH, Lewandowski RJ, Shea LD, Larson AC. Poly(lactide-co-glycolide) microspheres for MRI-monitored delivery of sorafenib in a rabbit VX2 model. Biomaterials. 2015; 61:299–306. PMID: 26022791.
Article21. Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, Gacchina CE, Sharma KV, Frenkel V, Wood BJ, Dreher MR. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release. 2012; 158:487–494. PMID: 22210162.
Article22. Anayama T, Nakajima T, Dunne M, Zheng J, Allen C, Driscoll B, Vines D, Keshavjee S, Jaffray D, Yasufuku K. A novel minimally invasive technique to create a rabbit VX2 lung tumor model for nano-sized image contrast and interventional studies. PLoS One. 2013; 8:e67355. PMID: 23840673.
Article23. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996; 86:353–364. PMID: 8756718.
Article24. Leader P, Månsson S, Besjakov TEJ. CT and MR imaging of the liver using liver specific contrast media. A comparative study in a tumor model. Acta Radiol. 1996; 37:242–249. PMID: 8845249.25. Guan L. Angiogenesis dependent characteristics of tumor observed on rabbit VX2 hepatic carcinoma. Int J Clin Exp Pathol. 2015; 8:12014–12027. PMID: 26722387.26. Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991; 83:288–291. PMID: 1671606.
Article27. Saloustros E, Georgoulias V. Docetaxel in the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2008; 8:1207–1222. PMID: 18699760.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Rabbit Brain Tumor Model Using VX2 Cells and Verification with the MRI in Neuroradiologic Research
- Intrahepatic Transneedle Inoculation of VX2 Particles for Obtaining a Solitary Hepatic Tumor in an Animal Model
- Development of Animal Model for Orthotopic Non-Small Cell Lung Cancer in Nude Rat
- Ultra Long Construct Minimally Invasive Spinal Stabilization Using Percutaneous Pedicle Screws in the Treatment of Symptomatic Multicentric Spinal Metastasis
- Percutaneous Radiofrequency Thermal Ablation of Lung VX2 Tumors in a Rabbit Model: Evaluation with Helical CT Findings for the Complete and Partal Ablation