Nat Prod Sci.
2018 Mar;24(1):36-39. 10.20307/nps.2018.24.1.36.
Content Analysis of Rutin in the Leaves of Boehmeria nivea Harvested in Different Regions of South Korea by HPLC-UV
- Affiliations
-
- 1Department of Integrative Plant Science, Chung-Ang University, Anseong 17546, Korea. slee@cau.ac.kr
- 2Yeong-Gwang Agricultural Technology Center, Yeonggwang 57031, Korea.
- 3Life Sciences Research Institute, Biomedic Co., Ltd., Bucheon 14548, Korea.
- 4Department of Food Science and Nutrition, Pusan National University, Busan 46241, Korea.
- KMID: 2409607
- DOI: http://doi.org/10.20307/nps.2018.24.1.36
Abstract
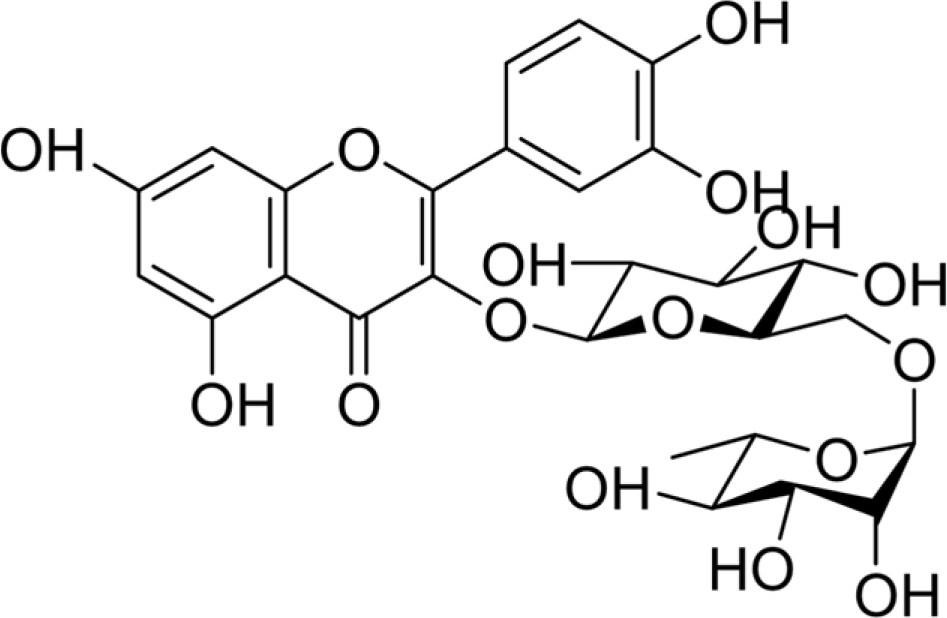
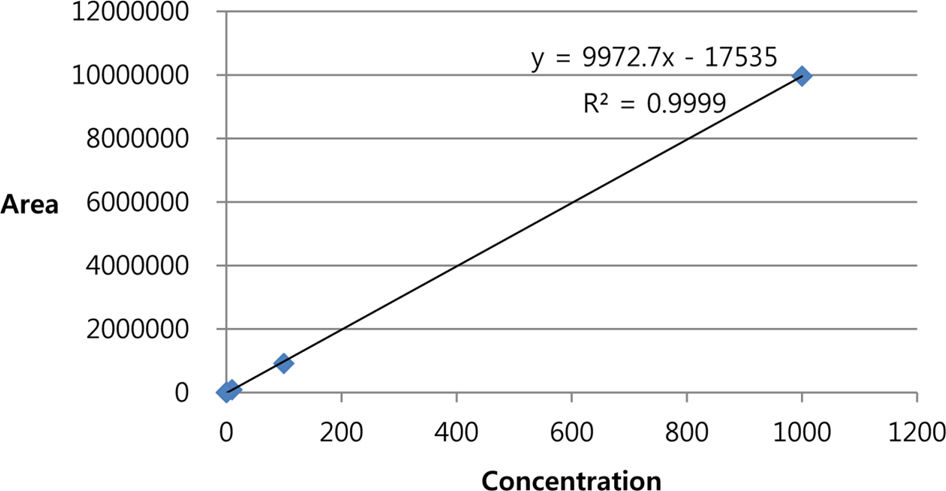
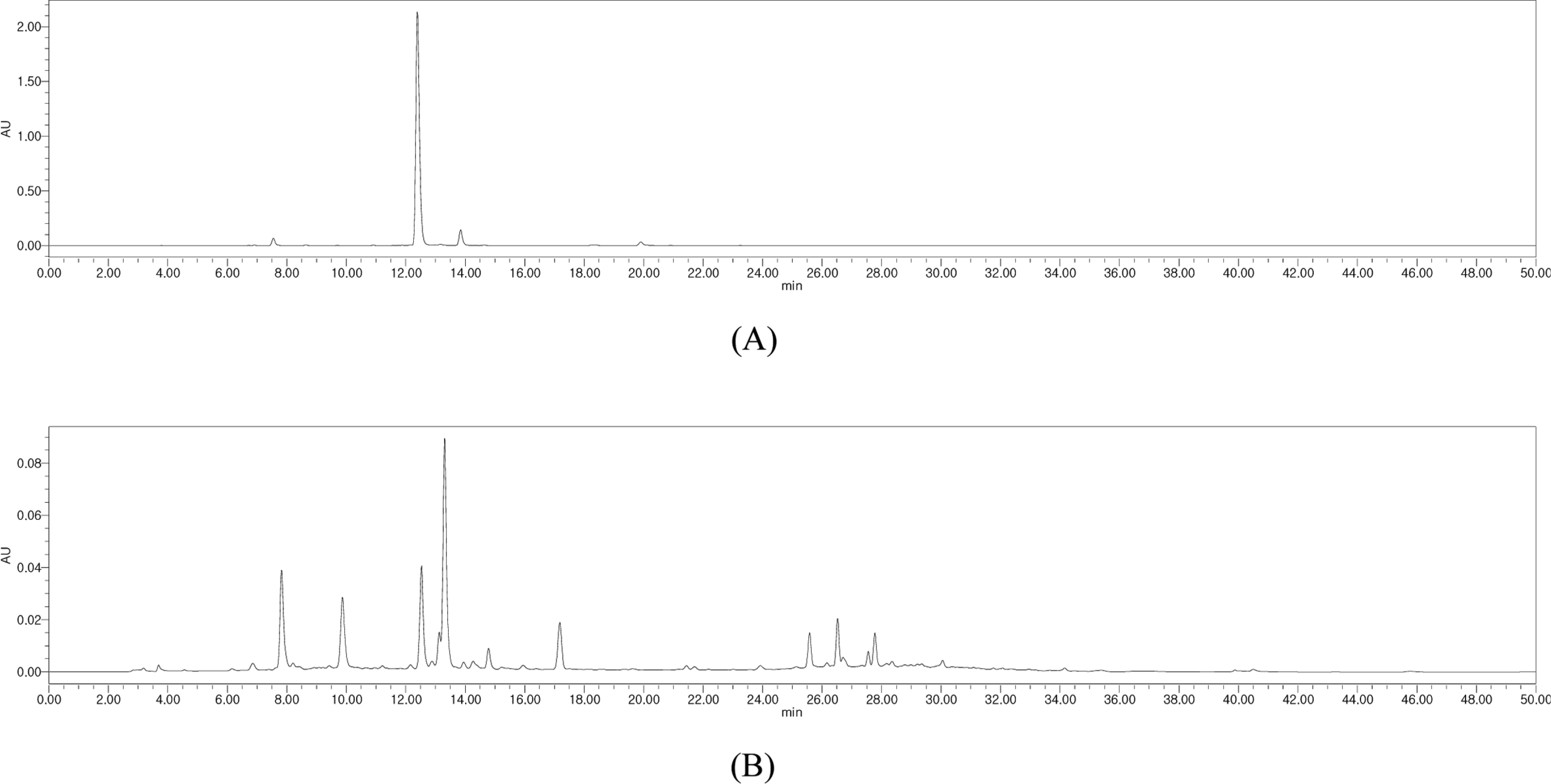
- Phytochemical analysis of Boehmeria nivea (Bn) leaves by medium pressure liquid chromatography led to the isolation of a flavonoid glycoside identified by spectroscopic analysis as rutin. The amount of rutin in the leaves of Bn harvested from nine regions in South Korea (Bn 1-9) which were collected on the months of June, July, August, and September was determined by HPLC-UV analysis. A gradient elution program that utilizes a Discovery® C18 (4.6 × 250 mm, 5 µm) column and mobile phase composed of 1% acetic acid-water: acetonitrile (90:10 to 60:40 for min) was followed. The injection volume and flow rate were 10 µl and 1 mL/ min, respectively. UV detection was set at 350 nm. Results show that Bn-8 harvested in September reported the highest content of rutin among the samples analyzed. This study provides a basis for the optimal harvest time of Bn which maximizes the yield of rutin.
Keyword
Figure
Reference
-
References
(1). Angelini L. G.., Lazzeri A.., Levita G.., Fontanelli D.., Bozzi C.Ind. Crops Prod. 2000. 11:145–161.(2). Paiva Junior C. Z.., de Carvalho L. H.., Fonseca V. M.., Monteiro S. N.., d'Almeida J. R. M.Polym. Test. 2004. 23:131–135.(3). Nam S.., Netravali A. N.Fibers Polym. 2006. 7:372–379.(4). Xu Q. -M.., Liu Y. -L.., Li X. -R.., Li X.., Yang S.-L. Nat. Prod. Res. 2011. 25:640–647.(5). Lin C. -C.., Yen M. -H.., Lo T. -S.., Linb J. -M. J.Ethnopharmacol. 1998. 60:9–17.(6). Sancheti S.., Sancheti S.., Bafna M.., Kim H. -R.., You Y. -H.., Seo S. -Y.Braz. J. Pharm. 2011. 21:146–154.(7). Sung M. J.., Davaatseren M.., Kim S. H.., Kim M. J.., Hwang J.-T. Pharm. Biol. 2013. 51:1131–1136.(8). Tan Z.., Wang C.., Yi Y.., Wang H.., Li M.., Zhou W.., Tan S.., Li F.Sep. Purif. Technol. 2014. 132:396–400.(9). Cho S.., Lee D. G.., Jung Y. -S.., Kim H. B.., Cho E. J.., Lee S.Nat. Prod. Sci. 2016. 22:134–139.(10). Couch J. F.., Naghski J.., Krewson C. F. J.Am. Chem. Soc. 1952. 74:424–425.(11). Ohsawa R.., Tsutsumi T.Euphytica. 1995. 86:183–189.
Article(12). Chua L. S. J.Ethnopharmacol. 2013. 150:805–817.(13). Kraujalis P.., Venskutonis P. R.., Ibáñez E.., Herrero M. J.Supercrit. Fluids. 2015. 104:234–242.(14). Erlund I.., Kosonen T.., Alfthan G.., Mäenpää J.., Perttunen K.., Kenraali J.., Parantainen J.., Aro A.Eur. J. Clin. Pharmacol. 2000. 56:545–553.(15). Hosseinzadeh H.., Nassiri-Asl M. J.Endocrinol. Invest. 2014. 37:783–788.
Article(16). Kitabayashi H.., Ujihara A.., Hirose T.., Minami M.Breeding Sci. 1995. 45:189–194.(17). Gevrenova R.., Kitanov G.., Ilieva D.Pharm. Biol. 2007. 45:149–155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phytochemical Identification from Boehmeria nivea Leaves and Analysis of (-)-Loliolide by HPLC
- Quantitative Analysis of the Flavonoid Content in the Leaves of Boehmeria nivea and Related Commercial Products
- Analysis of Phenolic Acid Content and Antioxidant Activity of Chestnut Honey from Different Regions of Korea
- Identification and HPLC Quantification of a Phytoecdysone and Three Phenolic Glycosides in Lamium takesimense Nakai
- The Effect of Rutin on the Melanogenesis and Nitric Oxide in UVB-irradiated HM3KO Human Melanoma