J Korean Acad Prosthodont.
2012 Jan;50(1):21-28.
Effects of implant collar design on marginal bone and soft tissue
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Pusan National University, Yangsan, Korea. jeonyc@paran.com
Abstract
- PURPOSE
The purpose of this study was to investigate the effects of implant collar design on marginal bone change and soft tissue response by an animal test
MATERIALS AND METHODS
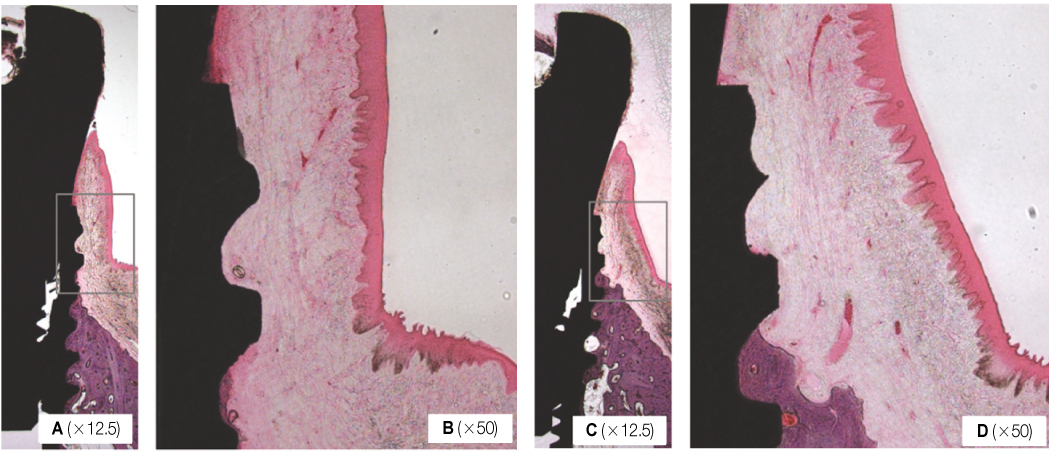
Two types of Implant (Neobiotech Co. Seoul, Korea) that only differs in collar design were planted on two healthy Beagle dogs. The implants were divided into two groups, the first group with a beveled collar (Bevel Group) and the second group with "S" shaped collar (Bioseal group). Standardized intraoral radiographs were used to investigate the mesio-distal change of the marginal bone. Histological analysis was done to evaluate the bucco-lingual marginal bone resorption and the soft tissue response adjacent to the implant. Mann-Whitney test was done to compare the mesio-distal marginal bone change at equivalent time for taking the radiographs and the tissue measurements between the groups.
RESULTS
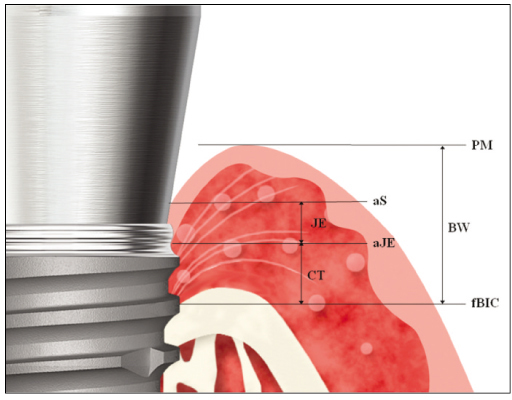
Radiographic and histological analysis showed that there was no difference in marginal bone change between the two groups (P>.05). Histological analysis showed Bioseal group had more rigid connective tissue attachment than the Bevel group. There was no difference in biological width (P>.05). Bevel group showed significantly longer junctional epithelium attachment and Bioseal group showed longer connective tissue attachment (P<.05).
CONCLUSION
For three months there were no differences in marginal bone change between the Bevel group and the Bioseal group. As for the soft tissue adjacent to the implant, Bioseal group showed longer connective tissue attachment while showing shorter junctional epithelium attachment. There were no differences in biologic width.
Keyword
MeSH Terms
Figure
Reference
-
1. Adell R, Lekholm U, Rockler B, Braånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981. 10:387–416.
Article2. Braånemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983. 50:399–410.
Article3. Linkow LI, Rinaldi AW, Weiss WW Jr, Smith GH. Factors influencing long-term implant success. J Prosthet Dent. 1990. 63:64–73.
Article4. Quirynen M, Naert I, van Steenberghe D. Fixture design and overload influence marginal bone loss and fixture success in the Braånemark system. Clin Oral Implants Res. 1992. 3:104–111.
Article5. Misch CE. Misch CE, editor. Stress factors: influence on treatment. Dental Implant Prosthetics. 2005. St. Louis: Mosby;71–90.6. Haider R, Watzek G, Plenk H. Effects of drill cooling and bone structure on IMZ implant fixation. Int J Oral Maxillofac Implants. 1993. 8:83–91.7. Isidor F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin Oral Implants Res. 1996. 7:143–152.
Article8. Duyck J, Rønold HJ, Van Oosterwyck H, Naert I, Vander Sloten J, Ellingsen JE. The influence of static and dynamic loading on marginal bone reactions around osseointegrated implants: an animal experimental study. Clin Oral Implants Res. 2001. 12:207–218.
Article9. Piattelli A, Ruggeri A, Franchi M, Romasco N, Trisi P. An histologic and histomorphometric study of bone reactions to unloaded and loaded non-submerged single implants in monkeys: a pilot study. J Oral Implantol. 1993. 19:314–320.10. Miyata T, Kobayashi Y, Araki H, Motomura Y, Shin K. The influence of controlled occlusal overload on peri-implant tissue: a histologic study in monkeys. Int J Oral Maxillofac Implants. 1998. 13:677–683.11. Miyata T, Kobayashi Y, Araki H, Ohto T, Shin K. The influence of controlled occlusal overload on peri-implant tissue. Part 3: A histologic study in monkeys. Int J Oral Maxillofac Implants. 2000. 15:425–431.12. Miyata T, Kobayashi Y, Araki H, Ohto T, Shin K. The influence of controlled occlusal overload on peri-implant tissue. part 4: a histologic study in monkeys. Int J Oral Maxillofac Implants. 2002. 17:384–390.13. Lekholm U, Ericsson I, Adell R, Slots J. The condition of the soft tissues at tooth and fixture abutments supporting fixed bridges. A microbiological and histological study. J Clin Periodontol. 1986. 13:558–562.
Article14. Adell R, Lekholm U, Rockler B, Braånemark PI, Lindhe J, Eriksson B, Sbordone L. Marginal tissue reactions at osseointegrated titanium fixtures (I). A 3-year longitudinal prospective study. Int J Oral Maxillofac Surg. 1986. 15:39–52.15. Rams TE, Roberts TW, Tatum H Jr, Keyes PH. The subgingival microbial flora associated with human dental implants. J Prosthet Dent. 1984. 51:529–534.
Article16. Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol. 1996. 23:971–973.17. Bae EK, Chung MK, Cha IH, Han DH. Marginal tissue response to different implant neck design. J Korean Acad Prosthodont. 2008. 46:602–609.
Article18. Hermann F, Lerner H, Palti A. Factors influencing the preservation of the periimplant marginal bone. Implant Dent. 2007. 16:165–175.
Article19. Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol. 1997. 68:186–198.
Article20. Hermann JS, Buser D, Schenk RK, Higginbottom FL, Cochran DL. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin Oral Implants Res. 2000. 11:1–11.
Article21. Tarnow DP, Cho SC, Wallace SS. The effect of inter-implant distance on the height of inter-implant bone crest. J Periodontol. 2000. 71:546–549.
Article22. Kim S, Oh KC, Han DH, Heo SJ, Ryu IC, Kwon JH, Han CH. Influence of transmucosal designs of three one-piece implant systems on early tissue responses: a histometric study in beagle dogs. Int J Oral Maxillofac Implants. 2010. 25:309–314.23. Gardner DM. Platform switching as a means to achieving implant esthetics. N Y State Dent J. 2005. 71:34–37.24. Lazzara RJ, Porter SS. Platform switching: a new concept in implant dentistry for controlling postrestorative crestal bone levels. Int J Periodontics Restorative Dent. 2006. 26:9–17.25. Maeda Y, Miura J, Taki I, Sogo M. Biomechanical analysis on platform switching: is there any biomechanical rationale? Clin Oral Implants Res. 2007. 18:581–584.
Article26. Quaresma SE, Cury PR, Sendyk WR, Sendyk C. A finite element analysis of two different dental implants: stress distribution in the prosthesis, abutment, implant, and supporting bone. J Oral Implantol. 2008. 34:1–6.
Article27. Kim DY, Kim TI, Seol YJ, Lee YM. Influence of platform switching on crestal bone resorption. J Korean Acad Periodontol. 2008. 38:135–142.
Article28. Lee SY, Piao CM, Koak JY, Kim SK, Kim YS, Ku Y, Rhyu IC, Han CH, Heo SJ. A 3-year prospective radiographic evaluation of marginal bone level around different implant systems. J Oral Rehabil. 2010. 37:538–544.
Article29. Yun HJ, Park JC, Yun JH, Jung UW, Kim CS, Choi SH, Cho KS. A short-term clinical study of marginal bone level change around microthreaded and platform-switched implants. J Periodontal Implant Sci. 2011. 41:211–217.
Article30. de Almeida FD, Carvalho AC, Fontes M, Pedrosa A, Costa R, Noleto JW, Mouraão CF. Radiographic evaluation of marginal bone level around internal-hex implants with switched platform: a clinical case report series. Int J Oral Maxillofac Implants. 2011. 26:587–592.31. Baumgarten H, Cocchetto R, Testori T, Meltzer A, Porter S. A new implant design for crestal bone preservation: initial observations and case report. Pract Proced Aesthet Dent. 2005. 17:735–740.32. Korean academy of oral and maxillofacial radiology. Intraoral radiography. Oral and Maxillofacial Radiology. 2001. 3rd ed. Seoul: Narae publishing;70–115.33. Baffone GM, Botticelli D, Pantani F, Cardoso LC, Schweikert MT, Lang NP. Influence of various implant platform configurations on peri-implant tissue dimensions: an experimental study in dog. Clin Oral Implants Res. 2011. 22:438–444.
Article34. Abrahamsson I, Berglundh T, Lindhe J. The mucosal barrier following abutment dis/reconnection. An experimental study in dogs. J Clin Periodontol. 1997. 24:568–572.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The influence of collar design on peri-implant marginal bone tissue
- Marginal tissue response to different Implant neck design
- Considerations in implant crestal module to preserve peri-implant tissue
- Improvement of peri-implant complications through customized prosthesis restoration allowing soft tissue space: a case report
- Influence of soft tissue and bone thickness on the dimensional change of peri-implant soft tissues: A clinical follow-up study