Ann Lab Med.
2018 Jul;38(4):324-330. 10.3343/alm.2018.38.4.324.
Recent Increase in the Incidence of TEM-135 β-Lactamase-harboring Neisseria gonorrhoeae in Korea
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. hmlee.labmed@gmail.com
- 2Department of Pharmacology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Medicine, Physician-Scientist Program, Yonsei University Graduate School of Medicine, Seoul, Korea.
- 4Department of Laboratory Medicine, The National Police Hospital, Seoul, Korea.
- KMID: 2408071
- DOI: http://doi.org/10.3343/alm.2018.38.4.324
Abstract
- BACKGROUND
We investigated the molecular epidemiological characteristics and antimicrobial susceptibility pattern of penicillinase-producing Neisseria gonorrhoeae (PPNG) isolates to monitor the change in distribution of bla(TEM) in Korea.
METHODS
We collected 804 PPNG isolates from diverse hospitals and clinics mainly located in Seoul, Korea, over a period of 11 years (2005-2015). Isolate susceptibility to seven antimicrobials was determined using the agar dilution test. The molecular epidemiological characteristics of the isolates were determined by Sanger sequencing of bla(TEM), N. gonorrhoeae multiantigen sequence typing (NG-MAST) and plasmid typing.
RESULTS
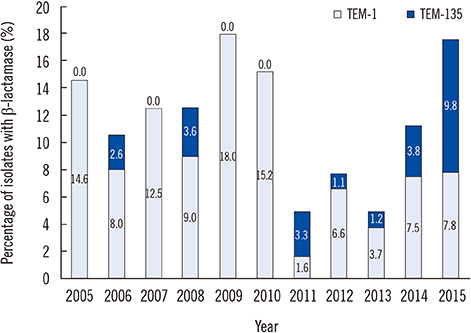
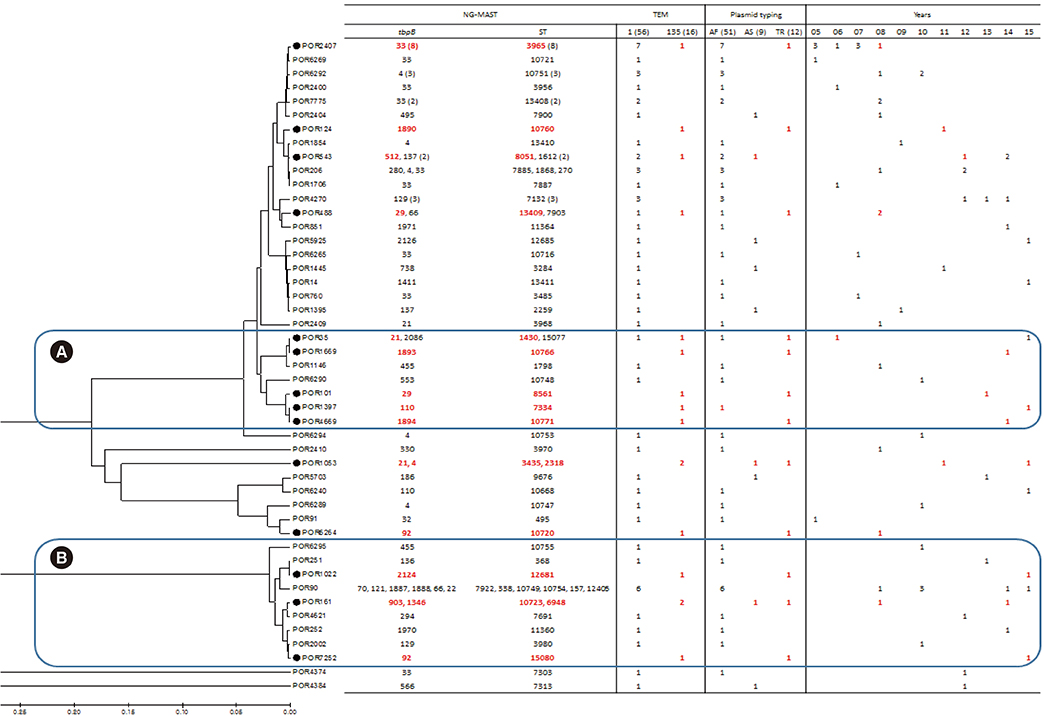
Among 72 fully sequenced PPNG isolates, sixteen (22.2%) possessed TEM-135. All TEM-135 isolates had a common silent mutation (c.18C>T), which was previously unreported. We observed a pattern of continuous increase in the number of TEM-135 isolates since 2012. The median and 90% minimum inhibitory concentration of azithromycin were substantially lower in the TEM-135 group than in the non-PPNG and TEM-1 groups. All TEM-135 isolates showed different NG-MAST types and predominantly harbored Toronto/Rio (75%) plasmids. A comprehensive comparative analysis of PPNG with TEM-135 according to NG-MAST, plasmid type, and year of isolation revealed a wide distribution.
CONCLUSIONS
The proportion of TEM-135 PPNG has continuously increased since 2012, in association with clonal spread. The difference at position 18 of the TEM-135 sequence can be interpreted as the existence of multiple clonal complexes. The possibility that TEM-135 was acquired via foreign plasmids requires careful follow-up and continuous monitoring of TEM-135 to ascertain whether it constitutes a step towards evolutionary change.
Keyword
MeSH Terms
Figure
Reference
-
1. Unemo M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014; 27:587–613.2. Ng LK, Martin I, Lau A. Trends of chromosomally mediated antimicrobial resistance in Neisseria gonorrhoeae in Canada: 1994–1999. Sex Transm Dis. 2003; 30:896–900.3. Ashford WA, Golash RG, Hemming VG. Penicillinase-producing Neisseria gonorrhoeae. Lancet. 1976; 2:657–658.4. Unemo M, Del Rio C, Shafer WM. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr. 2016; 4:EI10-0009-2015.
Article5. Bergstrom S, Norlander L, Norqvist A, Normark S. Contribution of a TEM-1-like beta-lactamase to penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978; 13:618–623.6. Pasquali F, Kehrenberg C, Manfreda G, Schwarz S. Physical linkage of Tn3 and part of Tn1721 in a tetracycline and ampicillin resistance plasmid from Salmonella typhimurium. J Antimicrob Chemother. 2005; 55:562–565.7. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests. CLSI supplement M02-A12. 12th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2015.8. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100-S25. 25th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2015.9. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0. 2015.10. Nakayama S, Tribuddharat C, Prombhul S, Shimuta K, Srifuengfung S, Unemo M, et al. Molecular analyses of TEM genes and their corresponding penicillinase-producing Neisseria gonorrhoeae isolates in Bangkok, Thailand. Antimicrob Agents Chemother. 2012; 56:916–920.11. Unemo M, Dillon JA. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin Microbiol Rev. 2011; 24:447–458.12. Palmer HM, Leeming JP, Turner A. A multiplex polymerase chain reaction to differentiate beta-lactamase plasmids of Neisseria gonorrhoeae. J Antimicrob Chemother. 2000; 45:777–782.13. Chen SC, Yin YP, Dai XQ, Yu RX, Han Y, Sun HH, et al. Prevalence and molecular epidemiological typing of penicillinase-producing Neisseria gonorrhoeae and their bla(TEM-135) gene variants in Nanjing, China. Sex Transm Dis. 2013; 40:872–876.14. Cole MJ, Unemo M, Grigorjev V, Quaye N, Woodford N. Genetic diversity of blaTEM alleles, antimicrobial susceptibility and molecular epidemiological characteristics of penicillinase-producing Neisseria gonorrhoeae from England and Wales. J Antimicrob Chemother. 2015; 70:3238–3243.15. Gianecini R, Oviedo C, Littvik A, Mendez E, Piccoli L, Montibello S, et al. Identification of TEM-135 β-lactamase in Neisseria gonorrhoeae strains carrying African and Toronto plasmids in Argentina. Antimicrob Agents Chemother. 2015; 59:717–720.16. Whiley D, Trembizki E, Buckley C, Freeman K, Lawrence A, Limnios A, et al. Penicillinase-producing plasmid types in Neisseria gonorrhoeae clinical isolates from Australia. Antimicrob Agents Chemother. 2014; 58:7576–7578.17. Ohnishi M, Ono E, Shimuta K, Watanabe H, Okamura N. Identification of TEM-135 β-lactamase in penicillinase-producing Neisseria gonorrhoeae strains in Japan. Antimicrob Agents Chemother. 2010; 54:3021–3023.18. Lee H, Kim H, Kim HJ, Suh YH, Yong D, Jeong SH, et al. Increasing incidence of high-level tetracycline-resistant Neisseria gonorrhoeae due to clonal spread and foreign import. Yonsei Med J. 2016; 57:350–357.19. Muhammad I, Golparian D, Dillon JA, Johansson A, Ohnishi M, Sethi S, et al. Characterisation of blaTEM genes and types of β-lactamase plasmids in Neisseria gonorrhoeae - the prevalent and conserved blaTEM-135 has not recently evolved and existed in the Toronto plasmid from the origin. BMC Infect Dis. 2014; 14:454.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of PPNG in Seoul, Korea ( 1983 ~ 1984 )
- Isolation of Beta-Lactamase-producing Neisseria gonorrhoeae
- Prevalence of Penicillinase-Producing Neisseria Gonorrhoeae (PPNG) in Seoul (1995)
- Antimicrobial Resistance of Neisseria gonorrhoeae Isolated in Korea
- Plasmid Patterns end in Vitro Susceptibility of Neisseria Gonorrhoeae in Taegu