Lab Anim Res.
2017 Jun;33(2):68-75. 10.5625/lar.2017.33.2.68.
Reproductive performance of genetically engineered mice housed in different housing systems
- Affiliations
-
- 1National Institute of Biologicals, Noida, India.
- 2National Brain Research Centre, Gurgaon, India.
- 3CSIR-Institute of Genomics and Integrative Biology, New Delhi, India. vp.singh@igib.res.in
- KMID: 2407418
- DOI: http://doi.org/10.5625/lar.2017.33.2.68
Abstract
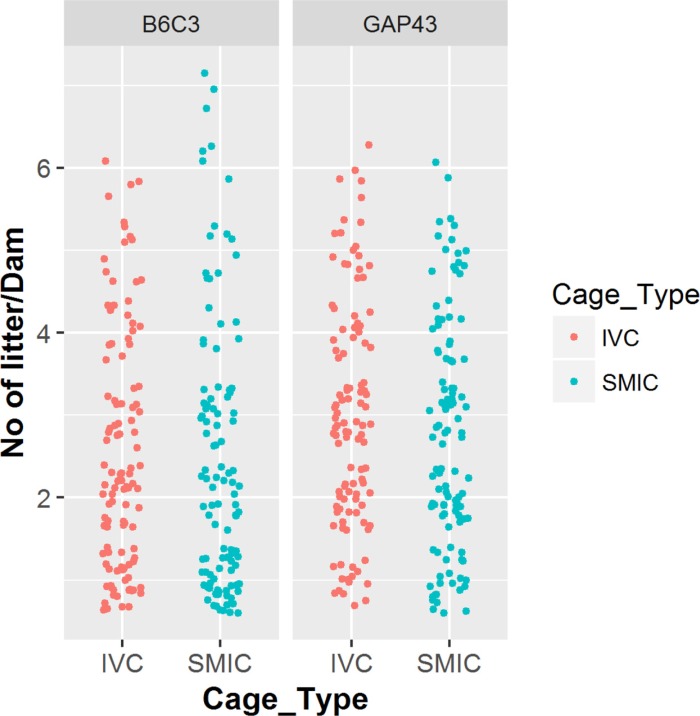
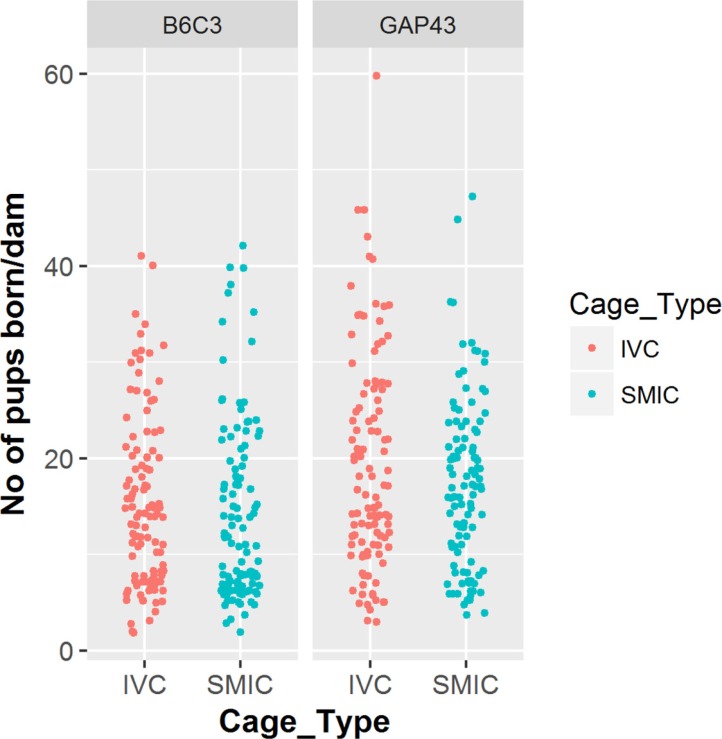
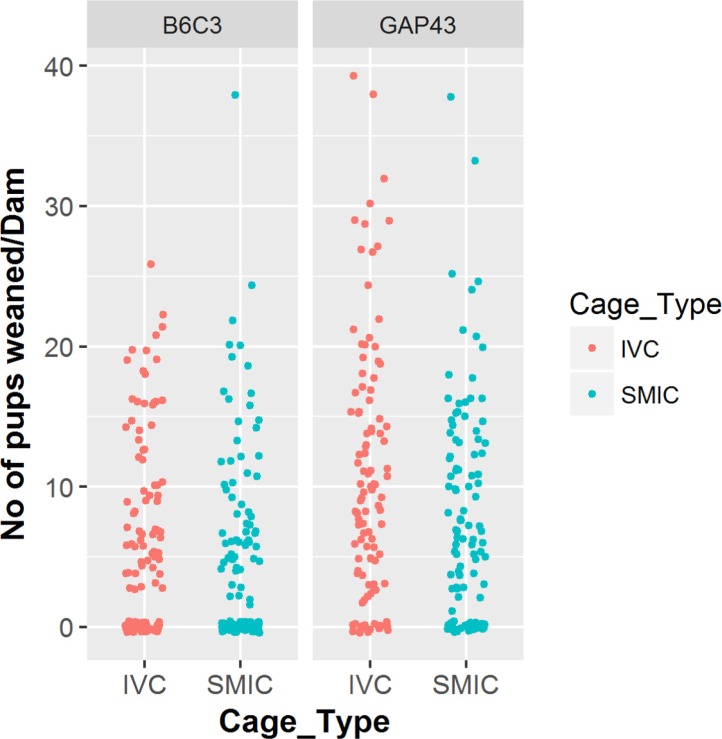
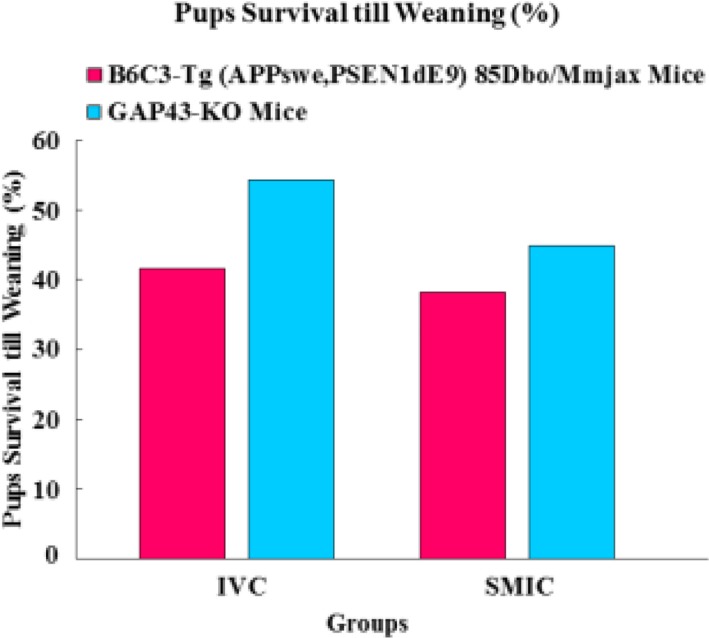
- The genetically engineered mice require special husbandry care and are mainly housed in Individually Ventilated Cage (IVC) systems and Static Micro Isolator Cages (SMIC) to minimize the risk for spreading undesirable microorganisms. However, the static micro isolation cage housing like SMIC are being replaced with IVC systems in many facilities due to a number of benefits like a higher density housing in limited space, better protection from biohazards and allergens and decreased work load due to decreased frequency of cage changing required in this system. The purpose of this study was to examine the reproductive performance of genetically engineered mice housed in individually ventilated cages (IVC) and Static Micro Isolator Cages (SMIC). When the B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax transgenic mice were housed in these two housing systems, the number of litters per dam, number of pups born per dam and number of pups weaned per dam were found to be slightly higher in the IVC as compared to the SMIC but the difference was not significant (P<0.05). In case of Growth Associated Protein 43 (GAP-43) knockout mice, the number of litters born per dam and the number of pups born per dam were marginally higher in the IVC as compared to those housed in SMIC but the difference was not significant (P<0.05). Only the number of pups weaned per dam were found to be significantly higher as compared to those housed in the SMIC system at P<0.05.
MeSH Terms
Figure
Reference
-
1. Brandstetter H1, Scheer M, Heinekamp C, Gippner-Steppert C, Loge O, Ruprecht L, Thull B, Wagner R, Wilhelm P, Scheuber HP. Working Group on Evaluation of IVC Systems of the Animal Welfare Information Center for Biomedical Research of the Faculty of Medicine. Performance evaluation of IVC systems. Lab Anim. 2005; 39(1):40–44. PMID: 15703123.
Article2. Baumans V, Schlingmann F, Vonck M, van Lith HA. Individually ventilated cages: beneficial for mice and men? Contemp Top Lab Anim Sci. 2002; 41(1):13–19.3. Reeb C, Jones R, Bearg D, Bedigan H, Myers D, Paigen B. Microenvironment in Ventilated Animal Cages with Differing Ventilation Rates, Mice Populations, and Frequency of Bedding Changes. Contemp Top Lab Anim Sci. 1998; 37(2):43–49.4. Sedlacek R, Orcutt R, Suit H, Rose E. Rose. Tokyo: Tokai University Press;1981. vol. 1:p. 151.5. Keller LS, White WJ, Snider MT, Lang CM. An evaluation of intra-cage ventilation in three animal caging systems. Lab Anim Sci. 1989; 39(3):237–242. PMID: 2724925.6. Lipman NS, Corning BF, Coiro MA Sr. The effects of intracage ventilation on microenvironmental conditions in filter-top cages. Lab Anim. 1992; 26(3):206–210. PMID: 1501435.7. Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fülöp L, Penke B, Zilberter Y, Harkany T, Pitkänen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009; 29(11):3453–3462. PMID: 19295151.8. Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK. Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet. 1997; 6(11):1951–1959. PMID: 9302276.
Article9. Krezowski J, Knudson D, Ebeling C, Pitstick R, Giri RK, Schenk D, Westaway D, Younkin L, Younkin SG, Ashe KH, Carlson GA. Identification of loci determining susceptibility to the lethal effects of amyloid precursor protein transgene overexpression. Hum Mol Genet. 2004; 13(18):1989–1997. PMID: 15254013.
Article10. Kosik KS, Orecchio LD, Bruns GA, Benowitz LI, MacDonald GP, Cox DR, Neve RL. Human GAP-43: its deduced amino acid sequence and chromosomal localization in mouse and human. Neuron. 1988; 1(2):127–132. PMID: 3272162.11. Metz GA, Schwab ME. Behavioral characterization in a comprehensive mouse test battery reveals motor and sensory impairments in growth-associated protein-43 null mutant mice. Neuroscience. 2004; 129(3):563–574. PMID: 15541878.
Article12. Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995; 80(3):445–452. PMID: 7859286.
Article13. Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci. 2002; 22(1):239–247. PMID: 11756507.
Article14. Maier DL, Mani S, Donovan SL, Soppet D, Tessarollo L, McCasland JS, Meiri KF. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci USA. 1999; 96(16):9397–9402. PMID: 10430954.
Article15. Sretavan DW, Kruger K. Randomized retinal ganglion cell axon routing at the optic chiasm of GAP-43-deficient mice: association with midline recrossing and lack of normal ipsilateral axon turning. J Neurosci. 1998; 18(24):10502–10513. PMID: 9852588.
Article16. Rekart JL, Quinn B, Mesulam MM, Routtenberg A. Subfieldspecific increase in brain growth protein in postmortem hippocampus of Alzheimer's patients. Neuroscience. 2004; 126(3):579–584. PMID: 15183507.
Article17. Rekart JL, Meiri K, Routtenberg A. Hippocampal-dependent memory is impaired in heterozygous GAP-43 knockout mice. Hippocampus. 2005; 15(1):1–7. PMID: 15390153.
Article18. Laber K, Veatch LM, Lopez MF, Mulligan JK, Lathers DM. Effects of housing density on weight gain, immune function, behavior, and plasma corticosterone concentrations in BALB/c and C57BL/6 mice. J Am Assoc Lab Anim Sci. 2008; 47(2):16–23.19. Lipman NS. Isolator Rodent Caging Systems (State of the Art): A Critical View. Contemp Top Lab Anim Sci. 1999; 38(5):9–17. PMID: 12086409.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cryopreservation of mouse resources
- Effects of housing conditions on stress, depressive like behavior and sensory‑motor performances of C57BL/6 mice
- Microbiological Contamination of Laboratory Mice and Rats in Korea from 2007 to 2008
- Effects of single housing on behavior, corticosterone level and body weight in male and female mice
- Insights into granulosa cell tumors using spontaneous or genetically engineered mouse models