Endocrinol Metab.
2015 Mar;30(1):105-109. 10.3803/EnM.2015.30.1.105.
Celiac Disease in a Predisposed Subject (HLA-DQ2.5) with Coexisting Graves' Disease
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul, Korea. imdrjs@khu.ac.kr
- KMID: 2407112
- DOI: http://doi.org/10.3803/EnM.2015.30.1.105
Abstract
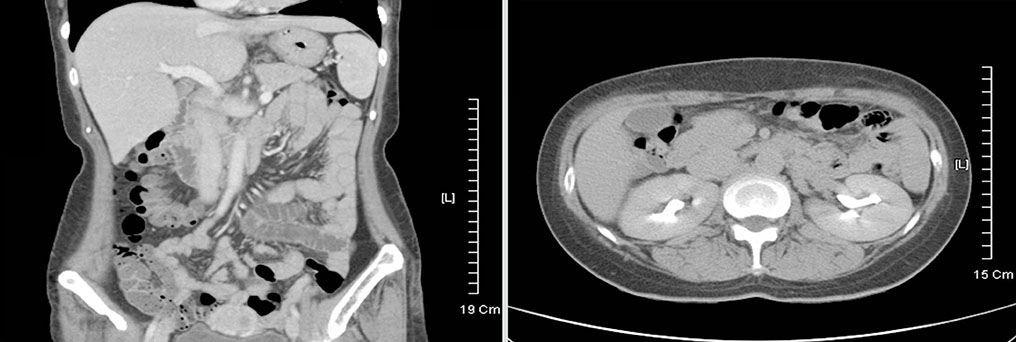
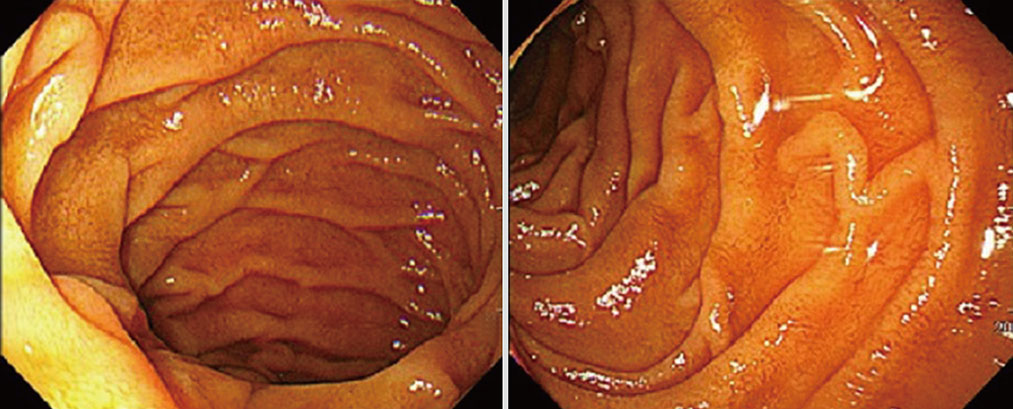
- Celiac disease is an intestinal autoimmune disorder, triggered by ingestion of a gluten-containing diet in genetically susceptible individuals. The genetic predisposition is related to human leukocyte antigen (HLA) class II genes, especially HLA-DQ2-positive patients. The prevalence of celiac disease has been estimated to be ~1% in Europe and the USA, but it is rarer and/or underdiagnosed in Asia. We report a case of celiac disease in a predisposed patient, with a HLA-DQ2 heterodimer, and Graves' disease that was treated successfully with a gluten-free diet. A 47-year-old woman complained of persistent chronic diarrhea and weight loss over a 9 month period. Results of all serological tests and stool exams were negative. However, the patient was found to carry the HLA DQ2 heterodimer. Symptoms improved after a gluten-free diet was initiated. The patient has been followed and has suffered no recurrence of symptoms while on the gluten-free diet. An overall diagnosis of celiac disease was made in a genetically predisposed patient (HLA-DQ2 heterodimer) with Graves' disease.
Keyword
MeSH Terms
Figure
Reference
-
1. Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002; 346:180–188.2. Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007; 117:41–49.3. Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001; 120:636–651.4. Wolters VM, Wijmenga C. Genetic background of celiac disease and its clinical implications. Am J Gastroenterol. 2008; 103:190–195.5. Megiorni F, Pizzuti A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: practical implications of the HLA molecular typing. J Biomed Sci. 2012; 19:88.6. Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, Ciclitira PJ, Sollid LM, Partanen J. European Genetics Cluster on Celiac Disease. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003; 64:469–477.7. Megiorni F, Mora B, Bonamico M, Barbato M, Montuori M, Viola F, Trabace S, Mazzilli MC. HLA-DQ and susceptibility to celiac disease: evidence for gender differences and parent-of-origin effects. Am J Gastroenterol. 2008; 103:997–1003.8. National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28-30, 2004. Gastroenterology. 2005; 128:4 Suppl 1. S1–S9.9. Ciacci C, Cavallaro R, Iovino P, Sabbatini F, Palumbo A, Amoruso D, Tortora R, Mazzacca G. Allergy prevalence in adult celiac disease. J Allergy Clin Immunol. 2004; 113:1199–1203.10. Megiorni F, Mora B, Bonamico M, Barbato M, Nenna R, Maiella G, Lulli P, Mazzilli MC. HLA-DQ and risk gradient for celiac disease. Hum Immunol. 2009; 70:55–59.11. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007; 357:1731–1743.12. Fasano A, Catassi C. Clinical practice: celiac disease. N Engl J Med. 2012; 367:2419–2426.13. Bai JC, Fried M, Corazza GR, Schuppan D, Farthing M, Catassi C, Greco L, Cohen H, Ciacci C, Eliakim R, Fasano A, Gonzalez A, Krabshuis JH, LeMair A. World Gastroenterology Organization. World Gastroenterology Organisation global guidelines on celiac disease. J Clin Gastroenterol. 2013; 47:121–126.14. Gannage MH, Abikaram G, Nasr F, Awada H. Osteomalacia secondary to celiac disease, primary hyperparathyroidism, and Graves' disease. Am J Med Sci. 1998; 315:136–139.15. Gweon TG, Lim CH, Byeon SW, Baeg MK, Lee JY, Moon SJ, Kim JS, Choi MG. A case of celiac disease. Korean J Gastroenterol. 2013; 61:338–342.16. Khandwala HM, Chibbar R, Bedi A. Celiac disease occurring in a patient with hypoparathyroidism and autoimmune thyroid disease. South Med J. 2006; 99:290–292.17. Papadopoulos KI, Hallengren B. Polyglandular autoimmune syndrome type III associated with coeliac disease and sarcoidosis. Postgrad Med J. 1993; 69:72–75.18. Zwolinska-Wcisło M, Galicka-Latała D, Sosin-Rudnicka L, Mach T, Rozpondek P. Celiac disease and other immunomediated diseases coexistance and influence of clinical course: case report. Przegl Lek. 2009; 66:466–468.19. Leffler D, Schuppan D, Pallav K, Najarian R, Goldsmith JD, Hansen J, Kabbani T, Dennis M, Kelly CP. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013; 62:996–1004.20. Coburn JA, Vande Voort JL, Lahr BD, Van Dyke CT, Kroning CM, Wu TT, Gandhi MJ, Murray JA. Human leukocyte antigen genetics and clinical features of self-treated patients on a gluten-free diet. J Clin Gastroenterol. 2013; 47:828–833.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Leukocyte Antigen-DQ Genotyping in Pediatric Celiac Disease
- Self-reported Wheat Sensitivity in Irritable Bowel Syndrome and Healthy Subjects: Prevalence of Celiac Markers and Response to Wheat-free Diet
- Prevalence of Anti-deamidated Gliadin Peptide Antibodies in Asian Patients With Irritable Bowel Syndrome
- Analysis of the markers related with relapse after withdrawal of antithyroid drug in patients with Graves' disease
- Insulin autoimmune syndrome induced by methimazole in a Korean girl with Graves' disease