Ann Surg Treat Res.
2018 Mar;94(3):118-128. 10.4174/astr.2018.94.3.118.
Effects of splanchnic vasoconstrictors on liver regeneration and survival after 90% rat hepatectomy
- Affiliations
-
- 1Department of Surgery, Korea University College of Medicine, Seoul, Korea. kimds1@korea.ac.kr
- 2Department of Biomedical Science, Korea University College of Medicine Graduate School, Seoul, Korea.
- 3Department of Pathology, Korea University College of Medicine, Seoul, Korea.
- KMID: 2405323
- DOI: http://doi.org/10.4174/astr.2018.94.3.118
Abstract
- PURPOSE
Posthepatectomy liver failure is a serious complication and considered to be caused by increased portal pressure and flow. Splanchnic vasoactive agents and propranolol are known to decrease portal pressure. The aim of this study was to identify optimal candidates with potential for clinical use among somatostatin, terlipressin, and propranolol using rats with 90% hepatectomy.
METHODS
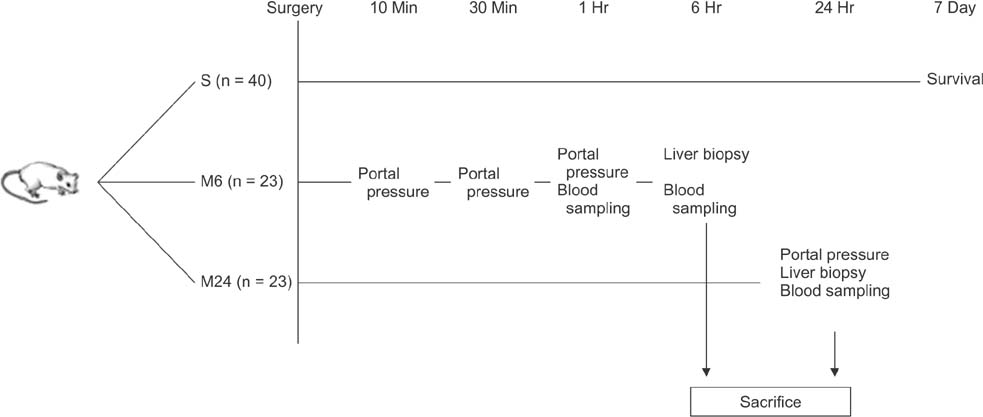
Rats were divided into 5 groups: sham operation (n = 6), control (n = 20), propranolol (n = 20), somatostatin (n = 20), and terlipressin group (n = 20). Seven-day survival rates and portal pressure change were measured, and biochemical, histologic, and molecular analyses were performed.
RESULTS
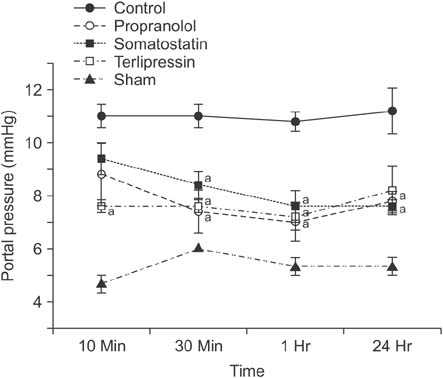
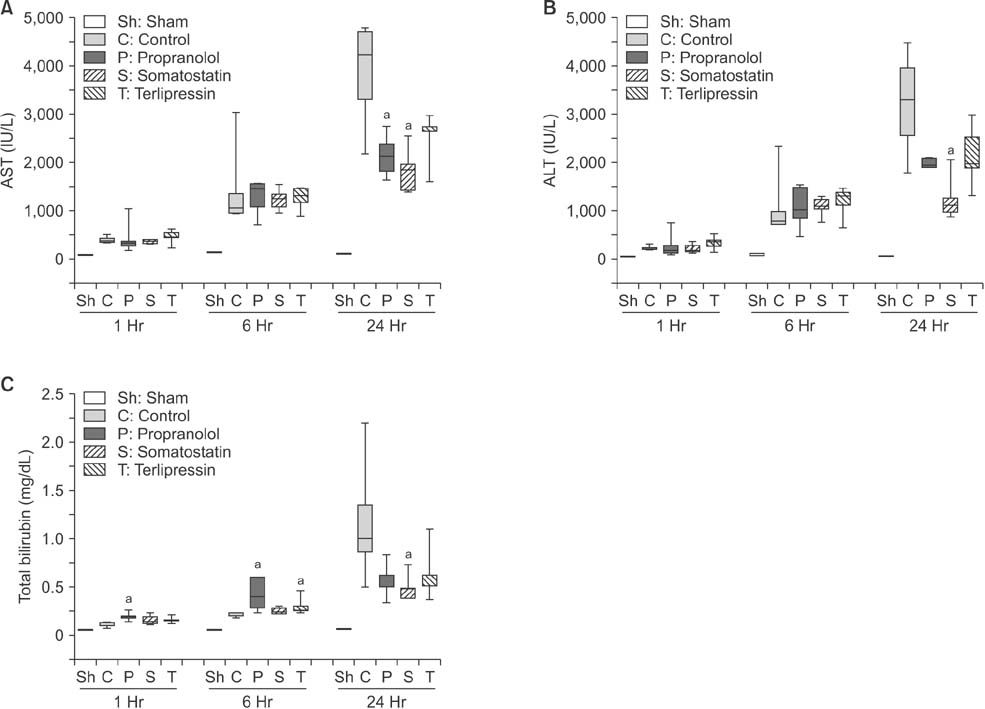
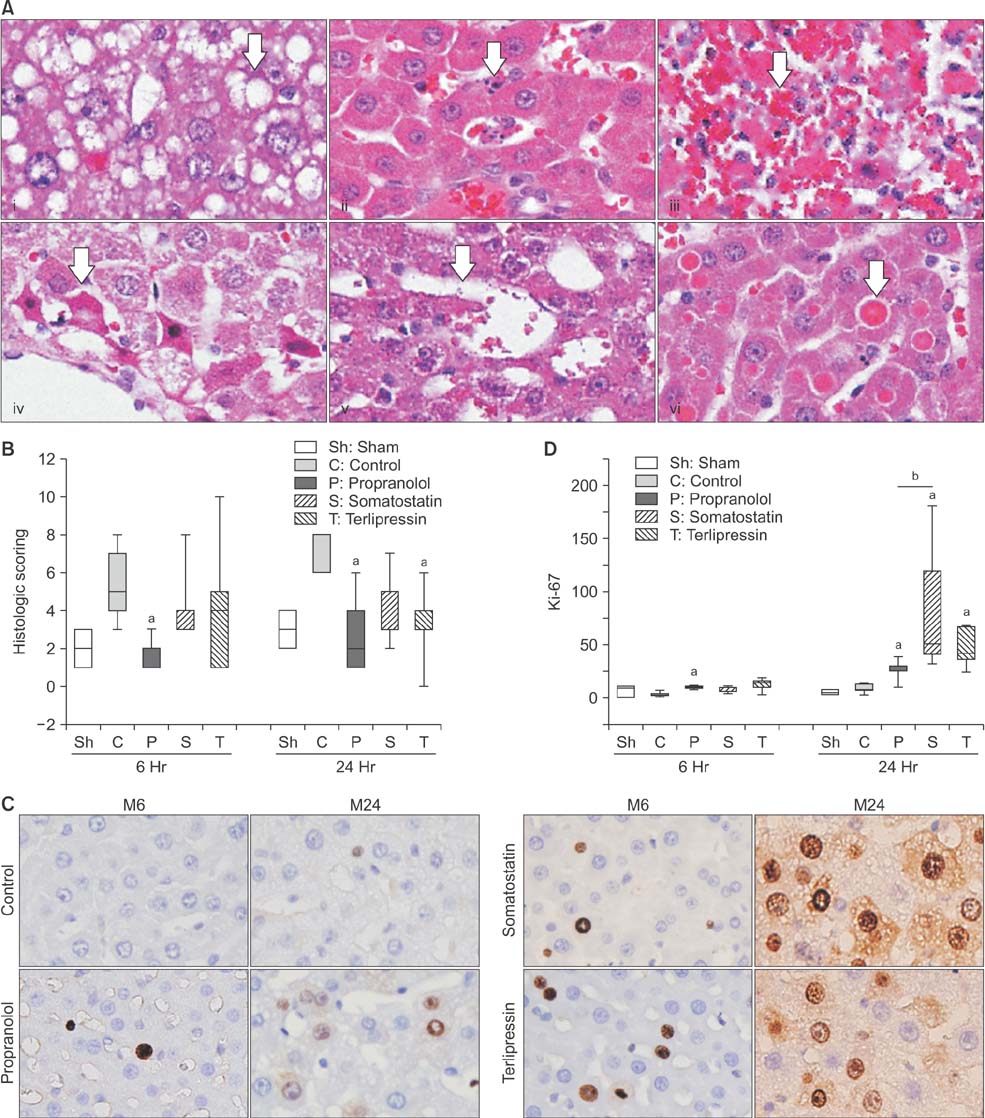
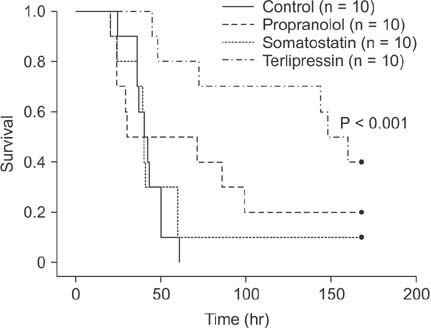
Portal pressure was significantly decreased in all 3 treatment groups compared to control. All treatment groups showed a tendency of decreased liver injury markers, and somatostatin showed the most prominent effect at 24 hours postoperatively. Histologic liver injury at 24 hours was significantly decreased in propranolol and terlipressin groups (P = 0.016, respectively) and somatostatin group showed borderline significance (P = 0.056). Hepatocyte proliferation was significantly increased after 24 hours in all treatment groups. Median survival was significantly increased in terlipressin group compared to control group (P < 0.01).
CONCLUSION
Terlipressin is considered as the best candidate, while somatostatin has good potential for clinical use, considering their effects on portal pressure and subsequent decrease in liver injury and increase in liver regeneration.
MeSH Terms
Figure
Reference
-
1. Helling TS. Liver failure following partial hepatectomy. HPB (Oxford). 2006; 8:165–174.
Article2. Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005; 5:2605–2610.
Article3. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011; 149:713–724.
Article4. Golriz M, Majlesara A, El Sakka S, Ashrafi M, Arwin J, Fard N, et al. Small for Size and Flow (SFSF) syndrome: an alternative description for posthepatectomy liver failure. Clin Res Hepatol Gastroenterol. 2016; 40:267–275.
Article5. Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997; 121:142–149.
Article6. Mukhtar A, Dabbous H. Modulation of splanchnic circulation: role in perioperative management of liver transplant patients. World J Gastroenterol. 2016; 22:1582–1592.
Article7. Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008; 134:1360–1368.
Article8. Baik SK, Jeong PH, Ji SW, Yoo BS, Kim HS, Lee DK, et al. Acute hemodynamic effects of octreotide and terlipressin in patients with cirrhosis: a randomized comparison. Am J Gastroenterol. 2005; 100:631–635.
Article9. Xu X, Man K, Zheng SS, Liang TB, Lee TK, Ng KT, et al. Attenuation of acute phase shear stress by somatostatin improves small-for-size liver graft survival. Liver Transpl. 2006; 12:621–627.
Article10. Yi NJ, Chang SH, Kwon CH, Cho JY, Yang EL, Suh KS, et al. Terlipressin effect of portal pressure control on liver regeneration in 90% hepatectomized rats. J Korean Surg Soc. 2005; 69:157–165.11. Kubota T, Takabe K, Yang M, Sekido H, Endo I, Ichikawa Y, et al. Minimum sizes for remnant and transplanted livers in rats. J Hep Bil Pancr Surg. 1997; 4:398–404.
Article12. Dahmen U, Madrahimov N, Madrahimova F, Ji Y, Schenk A, Dirsch O. Small-for-size syndrome in the rat: does size or technique matter? J Surg Res. 2008; 149:15–26.
Article13. Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today. 1997; 27:518–526.
Article14. Yamanaka K, Hatano E, Narita M, Kitamura K, Yanagida A, Asechi H, et al. Olprinone attenuates excessive shear stress through up-regulation of endothelial nitric oxide synthase in a rat excessive hepatectomy model. Liver Transpl. 2011; 17:60–69.
Article15. Gruttadauria S, Pagano D, Luca A, Gridelli B. Small-for-size syndrome in adult-to-adult living-related liver transplantation. World J Gastroenterol. 2010; 16:5011–5015.
Article16. Gonzalez HD, Liu ZW, Cashman S, Fusai GK. Small for size syndrome following living donor and split liver transplantation. World J Gastrointest Surg. 2010; 2:389–394.
Article17. Hessheimer AJ, Escobar B, Munoz J, Flores E, Gracia-Sancho J, Taura P, et al. Somatostatin therapy protects porcine livers in small-for-size liver transplantation. Am J Transplant. 2014; 14:1806–1816.
Article18. Ozden I, Kara M, Pinarbasi B, Salmaslioglu A, Yavru A, Kaymakoglu S, et al. Somatostatin and propranolol to treat small-for-size syndrome that occurred despite splenic artery ligation. Exp Clin Transplant. 2007; 5:686–689.19. Yu YD, Kim DS, Byun GY, Seo SO. Can propranolol be a viable option for the treatment of small-for-size syndrome? Liver Transpl. 2012; 18:747–748.
Article20. Higgins GM, Anderson RM. Experimental pathology of the liver I restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931; 12:186–202.21. Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg. 2006; 244:89–98.22. Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, et al. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008; 49:363–372.
Article23. Makino H, Togo S, Kubota T, Morioka D, Morita T, Kobayashi T, et al. A good model of hepatic failure after excessive hepatectomy in mice. J Surg Res. 2005; 127:171–176.
Article24. Gaub J, Iversen J. Rat liver regeneration after 90% partial hepatectomy. Hepatology. 1984; 4:902–904.
Article25. Debbaut C, De Wilde D, Casteleyn C, Cornillie P, Van Loo D, Van Hoorebeke L, et al. Modeling the impact of partial hepatectomy on the hepatic hemodynamics using a rat model. IEEE Trans Biomed Eng. 2012; 59:3293–3303.
Article26. Hatano E, Bennett BL, Manning AM, Qian T, Lemasters JJ, Brenner DA. NF-kappaB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis. Gastroenterology. 2001; 120:1251–1262.27. Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997; 276:60–66.
Article28. Kelly DM, Demetris AJ, Fung JJ, Marcos A, Zhu Y, Subbotin V, et al. Porcine partial liver transplantation: a novel model of the “small-for-size” liver graft. Liver Transpl. 2004; 10:253–263.
Article29. Reyes-Salcido V, Villalobos-Molina R. Evidence that dl-propranolol increases thymidine kinase activity, cell mitosis, and beta-adrenoceptors during rat liver regeneration. Arch Med Res. 2003; 34:273–275.30. Walldorf J, Hillebrand C, Aurich H, Stock P, Hempel M, Ebensing S, et al. Propranolol impairs liver regeneration after partial hepatectomy in C57Bl/6-mice by transient attenuation of hepatic lipid accumulation and increased apoptosis. Scand J Gastroenterol. 2010; 45:468–476.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of amino acid infusion on liver regeneration after partial hepatectomy in the rat with liver cirrhosis
- Effects of portal hyperperfusion on partial liver grafts in the presence of hyperdynamic splanchnic circulation: hepatic regeneration versus portal hyperperfusion injury
- Splenectomy affects the balance between hepatic growth factor and transforming growth factor-beta and its effect on liver regeneration is dependent on the amount of liver resection in rats
- The Effects of Intrasplenic Transplantation of Hepatocytes on Rats with Acute Liver Failure Induced by a 90% Hepatectomy
- The Effects of Korean Red Ginseng (Ginseng Radix Rubra) on Liver Regeneration after Partial Hepatectomy in Dogs