J Korean Surg Soc.
2012 Apr;82(4):238-245. 10.4174/jkss.2012.82.4.238.
Splenectomy affects the balance between hepatic growth factor and transforming growth factor-beta and its effect on liver regeneration is dependent on the amount of liver resection in rats
- Affiliations
-
- 1Department of Surgery, Kyung Hee University School of Medicine, Seoul, Korea. whipple@hanafos.com
- 2Department of Physiology, Kyung Hee University School of Medicine, Seoul, Korea.
- KMID: 1820072
- DOI: http://doi.org/10.4174/jkss.2012.82.4.238
Abstract
- PURPOSE
Small-for-size syndrome (SFSS) is a major problem in liver surgery, and splenectomy has been used to prevent SFSS. However, it is unknown whether splenectomy has the same effect on liver regeneration in both standard and marginal hepatectomy. The aim of this study is to see a difference in effect of splenectomy on liver regeneration according to the amount of liver resection.
METHODS
Thirty male Sprague-Dawley rats (220 to 260 g) were divided into the following five groups: control (n = 6), 70% hepatectomy (n = 6), 70% hepatectomy with splenectomy (n = 6), 90% hepatectomy (n = 6), and 90% hepatectomy with splenectomy (n = 6). The animals were euthanized 24 hours after surgery and liver specimens were obtained. To assess liver regeneration, we performed immunohistochemistry of liver tissue using 5-bromo-2-deoxyuridine (BrdU) labeling and Western blot analysis of hepatic growth factor (HGF) and transforming growth factor-beta (TGF-beta) in the liver tissue.
RESULTS
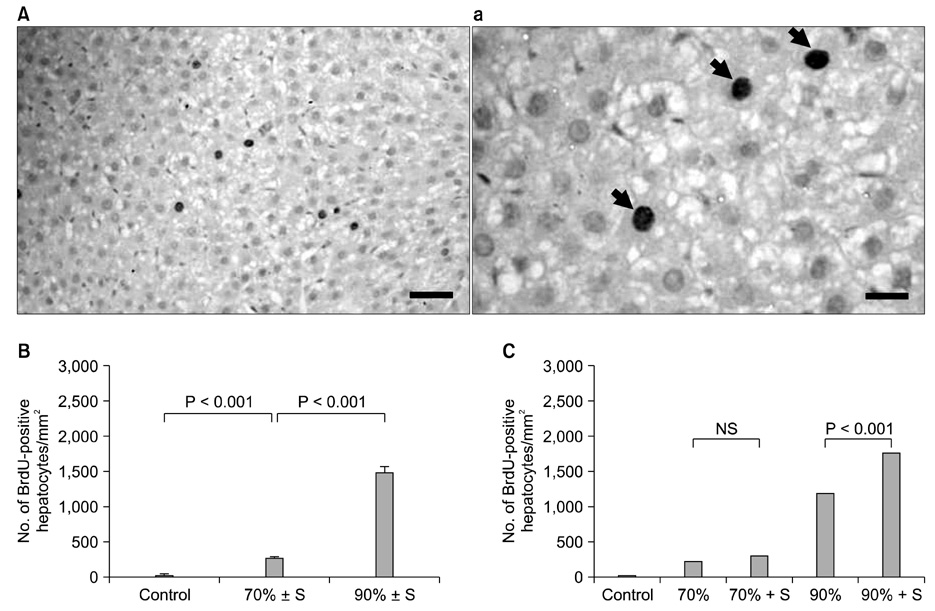
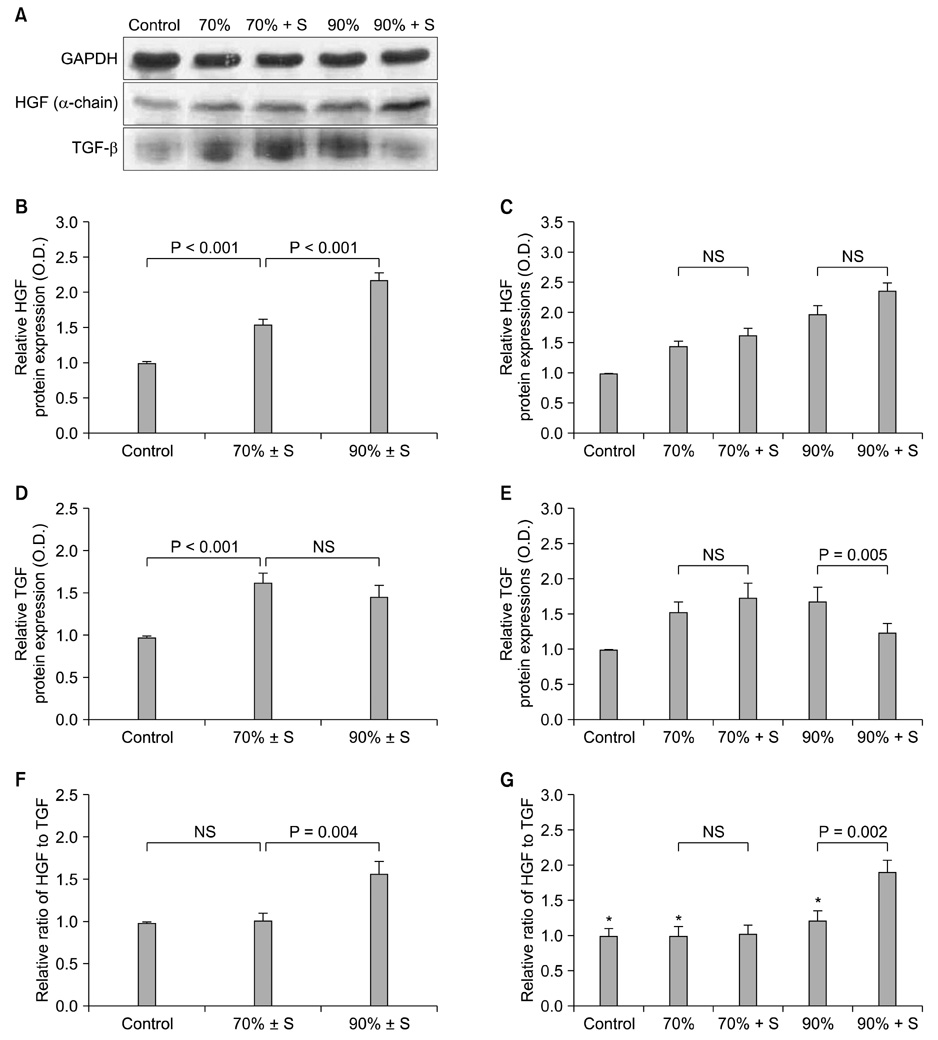
The splenectomized subgroup had a higher BrdU-positive cell count in the 90% hepatectomy group, but not in the 70% hepatectomy group (P < 0.001). Splenectomy significantly decreased TGF-beta expression (P = 0.005) and increased the HGF to TGF-beta ratio (P = 0.002) in the 90% hepatectomy group, but not in the 70% hepatectomy group.
CONCLUSION
The positive effect of splenectomy on liver regeneration was greater in the group with the larger liver resection. This phenomenon may be related to the relative balance between HGF and TGF-beta in the liver.
MeSH Terms
Figure
Reference
-
1. Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007. 356:1545–1559.2. Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005. 5:2605–2610.3. Marubashi S, Dono K, Miyamoto A, Takeda Y, Nagano H, Umeshita K, et al. Impact of graft size on postoperative thrombocytopenia in living donor liver transplant. Arch Surg. 2007. 142:1054–1058.4. Saner F, Sotiropoulos GC, Radtke A, Malago M, Broelsch CE, Herget-Rosenthal S. Small-for-size syndrome after living-donor liver transplantation treated by "portal vein wrapping" and single plasmapheresis. Transplantation. 2005. 79:625.5. Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, et al. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005. 5:1397–1404.6. Umeda Y, Yagi T, Sadamori H, Matsukawa H, Matsuda H, Shinoura S, et al. Effects of prophylactic splenic artery modulation on portal overperfusion and liver regeneration in small-for-size graft. Transplantation. 2008. 86:673–680.7. Arakawa Y, Shimada M, Uchiyama H, Ikegami T, Yoshizumi T, Imura S, et al. Beneficial effects of splenectomy on massive hepatectomy model in rats. Hepatol Res. 2009. 39:391–397.8. Sugawara Y, Yamamoto J, Shimada K, Yamasaki S, Kosuge T, Takayama T, et al. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000. 190:446–450.9. Eipel C, Glanemann M, Nuessler AK, Menger MD, Neuhaus P, Vollmar B. Ischemic preconditioning impairs liver regeneration in extended reduced-size livers. Ann Surg. 2005. 241:477–484.10. Kelly DM, Demetris AJ, Fung JJ, Marcos A, Zhu Y, Subbotin V, et al. Porcine partial liver transplantation: a novel model of the "small-for-size" liver graft. Liver Transpl. 2004. 10:253–263.11. Eipel C, Abshagen K, Ritter J, Cantré D, Menger MD, Vollmar B. Splenectomy improves survival by increasing arterial blood supply in a rat model of reduced-size liver. Transpl Int. 2010. 23:998–1007.12. Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931. 12:186–202.13. Gaub J, Iversen J. Rat liver regeneration after 90% partial hepatectomy. Hepatology. 1984. 4:902–904.14. Bucher NL. Jirtle RL, editor. Liver regeneration then and now. Liver regeneration and carcinogenesis: molecular and cellular mechanisms. 1995. San Diego: Academic Press;1–25.15. Kim J, Yi NJ, Shin WY, Kim T, Lee KU, Suh KS. Platelet transfusion can be related to liver regeneration after living donor liver transplantation. World J Surg. 2010. 34:1052–1058.16. Maetani Y, Itoh K, Egawa H, Shibata T, Ametani F, Kubo T, et al. Factors influencing liver regeneration following living-donor liver transplantation of the right hepatic lobe. Transplantation. 2003. 75:97–102.17. Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997. 276:60–66.18. Ichikawa T, Zhang YQ, Kogure K, Hasegawa Y, Takagi H, Mori M, et al. Transforming growth factor beta and activin tonically inhibit DNA synthesis in the rat liver. Hepatology. 2001. 34:918–925.19. Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010. 176:2–13.20. Ueda S, Yamanoi A, Hishikawa Y, Dhar DK, Tachibana M, Nagasue N. Transforming growth factor-beta1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab Invest. 2003. 83:1595–1603.21. Ito K, Ozasa H, Horikawa S. Effects of prior splenectomy on remnant liver after partial hepatectomy with Pringle maneuver in rats. Liver Int. 2005. 25:438–444.22. Ito K, Ozasa H, Noda Y, Horikawa S. Splenic artery ligation ameliorates hepatic ischemia and reperfusion injury in rats. Liver Int. 2006. 26:254–260.23. Glanemann M, Eipel C, Nussler AK, Vollmar B, Neuhaus P. Hyperperfusion syndrome in small-for-size livers. Eur Surg Res. 2005. 37:335–341.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transforming growth factor-beta1 protein, proliferation and apoptosis of oval cells in acetylaminofluorene-induced rat liver regeneration
- Expressions of transforming growth factor beta in patients with rheumatioid arthritis and osteoarthritis
- Correlation between Transforming Growth Factor-beta and Procollagen III with Regenerative Activity in Acute Liver Injury, and the Effect of Prostaglandin E2
- The Effect of Transforming Growth Factor-beta and Mannose-6-Phosphate on the Proliferation of Subconjunctival Fibroblast of Rabbit
- Expression of TGF-beta isoforms in the Human Fetal Hepatic Erythropoiesis