Cancer Res Treat.
2015 Oct;47(4):706-717. 10.4143/crt.2014.174.
Palliative Radiotherapy in the Presence of Well-Controlled Metastatic Disease after Initial Chemotherapy May Prolong Survival in Patients with Metastatic Esophageal and Gastric Cancer
- Affiliations
-
- 1Department of Clinical Oncology, Castle Hill Hospital, Hull and East Yorkshire, NHS Trust, Hull, UK. mohan.hingorani@hey.nhs.uk
- 2Hull and York Medical School, Hull, UK.
- 3Department of Clinical Oncology, St. James Institute of Oncology, Leeds Teaching Hospital, NHS Trust, Leeds, UK.
- 4Faculty of Science and Engineering, University of Hull, Hull, UK.
- 5Faculty of Health and Well-Being, University of Sheffield-Hallam, Sheffield, UK.
- KMID: 2403389
- DOI: http://doi.org/10.4143/crt.2014.174
Abstract
- PURPOSE
We report the outcomes of patients treated with palliative radiotherapy (pRT) to the primary tumour in the context of well-controlled metastatic disease after initial chemotherapy.
MATERIALS AND METHODS
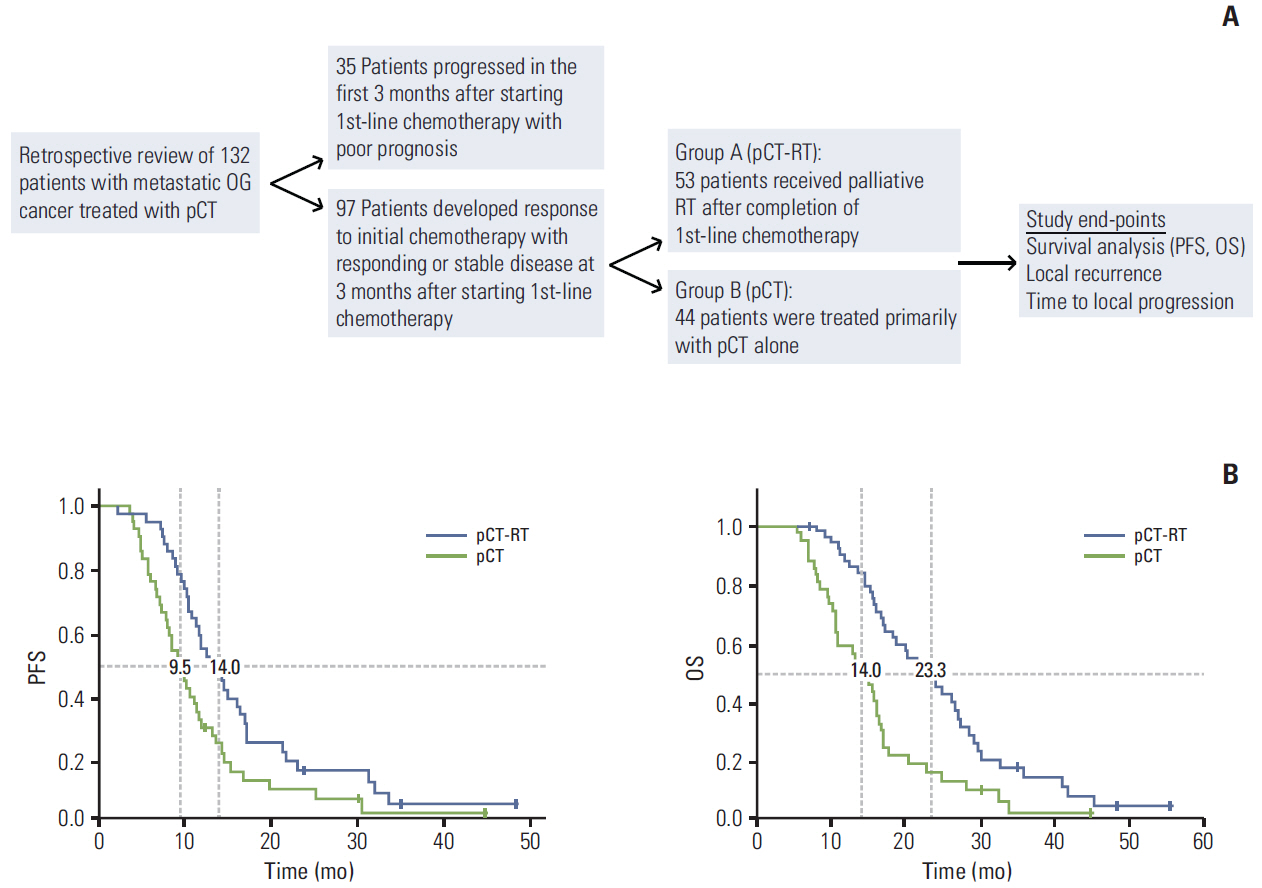
Clinical records of 132 patients with metastatic esophago-gastric (OG) cancer treated with palliative chemotherapy (pCT) between January 2009 and June 2013 were reviewed. Ninetyseven patients had responding or stable disease after 3 months of chemotherapy, of whom 53 patients received pRT to the primary tumour after initial chemotherapy in the presence of well-controlled metastatic disease (group A, pCT-RT). The remaining 44 patients were treated with pCT alone (group B, pCT). Treatment-related outcomes were assessed in above groups including time to local progression (TTLP), progression-free and overall survival.
RESULTS
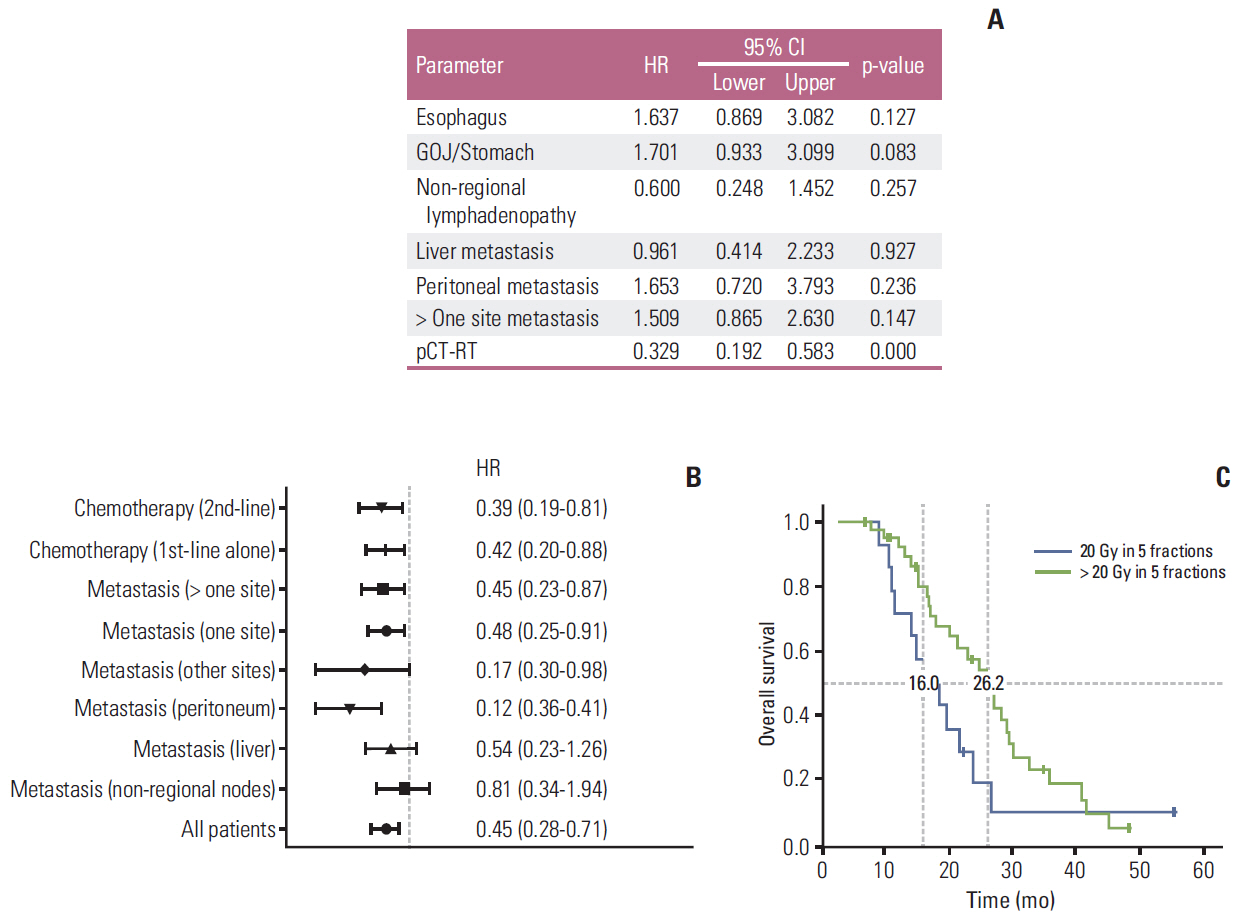
The median overall survival for patients treated with pRT after initial chemotherapy (group A) was 23.3 months (95% confidence interval [CI], 17.70 to 28.89 months) and significantly higher than the 14 months (95% CI, 10.91 to 17.08 months) in patients treated with pCT alone (group B) (p < 0.001). The use of pCT-RT was an independent predictor of OS in multivariate analysis. Local recurrence was observed in 12/53 of patients (23%) in group A compared to 16/44 (36%) in group B. The median TTLP was significantly higher in patients after pCT-RT at 17.3 months (5.23 months to 44.50 months) compared to 8.3 months (range, 4.10 to 25.23 months) in patients treated with pCT alone (p=0.006).
CONCLUSION
The possibility of pRT influencing systemic disease in advanced OG cancer has not been reported, and results from the present study present strong arguments for investigation of this therapeutic strategy in a randomized trial.
MeSH Terms
Figure
Cited by 1 articles
-
Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach
J Gastric Cancer. 2019;19(1):1-48. doi: 10.5230/jgc.2019.19.e8.
Reference
-
References
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: esophageal and esophagogastric junction cancers [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2013. [cited 2014 Dec 1]. Available from: http://www.nccn.org.2. Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008; 26:1435–42.
Article3. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.
Article4. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009; 20:666–73.
Article5. Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002; 20:1996–2004.
Article6. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–7.
Article7. Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008; 358:36–46.
Article8. Rueth NM, Shaw D, D'Cunha J, Cho C, Maddaus MA, Andrade RS. Esophageal stenting and radiotherapy: a multi-modal approach for the palliation of symptomatic malignant dysphagia. Ann Surg Oncol. 2012; 19:4223–8.
Article9. Eldeeb H, El-Hadaad HA. Radiotherapy versus stenting in treating malignant dysphagia. J Gastrointest Onco. 2012; 3:322–5.10. Zhong J, Wu Y, Xu Z, Liu X, Xu B, Zhai Z. Treatment of medium and late stage esophageal carcinoma with combined endoscopic metal stenting and radiotherapy. Chin Med J (Engl). 2003; 116:24–8.11. Hanna WC, Sudarshan M, Roberge D, David M, Waschke KA, Mayrand S, et al. What is the optimal management of dysphagia in metastatic esophageal cancer? Curr Oncol. 2012; 19:e60–6.
Article12. Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004; 364:1497–504.
Article13. Sharma V, Mahantshetty U, Dinshaw KA, Deshpande R, Sharma S. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002; 52:310–5.
Article14. Coia LR, Soffen EM, Schultheiss TE, Martin EE, Hanks GE. Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer. 1993; 71:281–6.
Article15. Urba SG, Turrisi AT 3rd. Split-course accelerated radiation therapy combined with carboplatin and 5-fluorouracil for palliation of metastatic or unresectable carcinoma of the esophagus. Cancer. 1995; 75:435–9.
Article16. Hayter CR, Huff-Winters C, Paszat L, Youssef YM, Shelley WE, Schulze K. A prospective trial of short-course radiotherapy plus chemotherapy for palliation of dysphagia from advanced esophageal cancer. Radiother Oncol. 2000; 56:329–33.
Article17. Harvey JA, Bessell JR, Beller E, Thomas J, Gotley DC, Burmeister BH, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus. 2004; 17:260–5.
Article18. Burmeister BH, Walpole ET, Burmeister EA, Thomas J, Thomson DB, Harvey JA, et al. Feasibility of chemoradiation therapy with protracted infusion of 5-fluorouracil for esophageal cancer patients not suitable for cisplatin. Int J Clin Oncol. 2005; 10:256–61.19. Cho SH, Shim HJ, Lee SR, Ahn JS, Yang DH, Kim YK, et al. Concurrent chemoradiotherapy with S-1 and cisplatin in advanced esophageal cancer. Dis Esophagus. 2008; 21:697–703.
Article20. Ikeda E, Kojima T, Kaneko K, Minashi K, Onozawa M, Nihei K, et al. Efficacy of concurrent chemoradiotherapy as a palliative treatment in stage IVB esophageal cancer patients with dysphagia. Jpn J Clin Oncol. 2011; 41:964–72.
Article21. Sreedharan A, Harris K, Crellin A, Forman D, Everett SM. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev. 2009; (4):CD005048.
Article22. Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, et al. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 2011; 137:125–30.23. Lee JA, Lim DH, Park W, Ahn YC, Huh SJ. Radiation therapy for gastric cancer bleeding. Tumori. 2009; 95:726–30.
Article24. Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998; 43:575–7.
Article25. Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2012; 2:191.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Metastatic Gastric Cancer
- Factors Affecting Long Term Survival for Metastatic Gastric Cancer Treated with Chemotherapy
- Role of Metastasectomy on Overall Survival of Patients with Metastatic Gastric Cancer
- Evaluating the recent developments in palliative chemotherapy for metastatic colorectal cancer
- Simultaneous Esophageal and Gastric Metastases from Lung Cancer