J Vet Sci.
2018 Jan;19(1):27-33. 10.4142/jvs.2018.19.1.27.
Piglet colibacillosis diagnosis based on multiplex polymerase chain reaction and immunohistochemistry of paraffin-embedded tissues
- Affiliations
-
- 1Department of Veterinary Pathology, School of Veterinary Medicine, Federal University of Rio Grande do Sul, Porto Alegre 91540-000, Brazil. cintiadelorenzobr@gmail.com
- 2State Foundation of Livestock Research, Eldorado do Sul 92990-000, Brazil.
- KMID: 2402692
- DOI: http://doi.org/10.4142/jvs.2018.19.1.27
Abstract
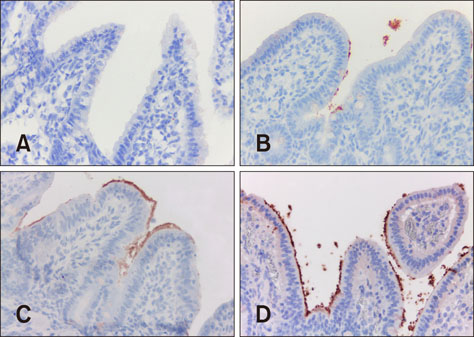
- Enterotoxigenic Escherichia coli (ETEC) causes diarrhea in pigs, referred to as colibacillosis. The aim of this study was to optimize multiplex polymerase chain reaction (PCR) and immunohistochemistry (IHC) analyses of paraffin-embedded material to detect pathogenic E. coli strains causing colibacillosis in pigs. Multiplex PCR was optimized for fimbriae (F18, F4, F6, F5, and F41) and toxins (types A and B heat-stable toxins [STaP and STb], heat-labile toxin [LT], and type 2 Shiga toxin [ST(x2e)]), and IHC was optimized for an anti-E. coli polyclonal antibody. Samples (132) from pigs received between 2006 and 2014 with clinical and histopathological diagnoses of colibacillosis were analyzed. E. coli was detected by IHC in 78.7%, and at least one virulence factor gene was detected in 71.2%. Pathogenic strains of ETEC with at least one fimbria and one toxin were detected in 40% of the samples in multiplex PCR. The most frequent virulence types were F18-STaP (7.5%), F18-STaP-STb (5.7%), and F4-STaP (3.8%). A statistically significant association was noted between virulence factors F4, F18, STaP, and STb and positive immunostaining results. Colibacillosis diagnosis through multiplex PCR and IHC of paraffin-embedded tissues is a practical approach, as samples can be fixed and stored for long periods before analysis.
MeSH Terms
Figure
Reference
-
1. Arslan A, Saglam YS, Temur A. Detection of rabies viral antigens in non-autolysed and autolysed tissues by using an immunoperoxidase technique. Vet Rec. 2004; 155:550–552.
Article2. Boerlin P, Travis R, Gyles CL, Reid-Smith R, Janecko N, Lim H, Nicholson V, McEwen SA, Friendship R, Archambault M. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl Environ Microbiol. 2005; 71:6753–6761.
Article3. Bosworth B, Casey T. Identification of toxin and pilus genes in porcine Escherichia coli using polymerase chain reaction (PCR) with multiple prime pairs. In : Abstracts of the 97th General Meeting of the American Society for Microbiology; 4-8 May, 1997; Miami Beach, USA.4. Do TN, Cu PH, Nguyen HX, Au TX, Vu QN, Driesen SJ, Townsend KM, Chin JJ, Trott DJ. Pathotypes and serogroups of enterotoxigenic Escherichia coli isolated from pre-weaning pigs in north Vietnam. J Med Microbiol. 2006; 55:93–99.
Article5. Fairbrother JM, Gyles CL. Colibacillosis. In : Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Ames: Blackwell Publishing;2012. p. 723–749.6. Frydendahl K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet Microbiol. 2002; 85:169–182.
Article7. Gelberg HB. Alimentary system and the peritoneum, omentum, mesentery and peritoneal cavity. In : Mcgavin MD, Zachary JF, editors. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis: Blackwell Publishing;2012. p. 374.8. Gilbert MT, Haselkorn T, Bunce M, Sanchez JJ, Lucas SB, Jewell LD, Van Marck E, Worobey M. The isolation of nucleic acids from fixed, paraffin-embedded tissues-which methods are useful when? PLoS One. 2007; 2:e537.
Article9. Gyles CL, Prescott JF, Songer JG, Thoen CO. Pathogenesis of Bacterial Infections in Animals. 3rd ed. Ames: Blackwell Publishing;2004.10. Hampson DJ. Post-weaning E. coli diarrhea in pigs. In : Gyles CL, editor. Esherichia coli in Domestic Animals and Humans. 1st ed. London: Cab International;1994. p. 171–192.11. Hunt JL, Dacic S. Applications in anatomic pathology. In : Cagle PT, Allen TC, editors. Basic Concepts of Molecular Pathology. 1st ed. New York: Springer-Verlag;2009. p. 69–72.12. Ikwap K, Larsson J, Jacobson M, Owiny DO, Nasinyama GW, Nabukenya I, Mattsson S, Aspan A, Erume J. Prevalence of adhesin and toxin genes in E. coli strains isolated from diarrheic and non-diarrheic pigs from smallholder herds in northern and eastern Uganda. BMC Microbiol. 2016; 16:178.
Article13. Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991; 4:80–128.
Article14. Macêdo NR, Menezes CPL, Lage AP, Ristow LE, Reis A, Guedes RMC. [Detection of pathogenic strains by multiplex PCR and antimicrobial sensitivity of Escherichia coli isolated from piglets]. Arq Bras Med Vet Zootec. 2007; 59:1117–1123. Portuguese.
Article15. Madoroba E, Van Driessche E, De Greve H, Mast J, Ncube I, Read J, Beeckmans S. Prevalence of enterotoxigenic Escherichia coli virulence genes from scouring piglets in Zimbabwe. Trop Anim Health Prod. 2009; 41:1539–1547.
Article16. Markey BK, Leonard F, Archambault M, Culinane A, Maguire D. Clinical Veterinary Microbiology. 2nd ed. St. Louis: Elsevier;2013.17. Moon HW, Hoffman LJ, Cornick NA, Booher SL, Bosworth BT. Prevalences of some virulence genes among Escherichia coli isolates from swine presented to a diagnostic laboratory in Iowa. J Vet Diagn Invest. 1999; 11:557–560.
Article18. Nagy B, Nagy G, Meder M, Mocsári E. Enterotoxigenic Escherichia coli, rotavirus, porcine epidemic diarrhoea virus, adenovirus and calici-like virus in porcine postweaning diarrhoea in Hungary. Acta Vet Hung. 1996; 44:9–19.19. Ngeleka M, Pritchard J, Appleyard G, Middleton DM, Fairbrother JM. Isolation and association of Escherichia coli AIDA-I/STb, rather than EAST1 pathotype, with diarrhea in piglets and antibiotic sensitivity of isolates. J Vet Diagn Invest. 2003; 15:242–252.
Article20. Ojeniyi B, Ahrens P, Meyling A. Detection of fimbrial and toxin genes in Escherichia coli and their prevalence in piglets with diarrhoea. The application of colony hybridization assay, polymerase chain reaction and phenotypic assays. Zentralbl Veterinarmed B. 1994; 41:49–59.
Article21. Post KW, Bosworth BT, Knoth JL. Frequency of virulence factors in Escherichia coli isolated from pigs with postweaning diarrhea and edema disease in North Carolina. Swine Health Prod. 2000; 8:119–120.22. Ramos-Vara JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005; 42:405–426.
Article23. Sato JPH, Takeuti KL, Andrade MR, Koerich PKV, Tagliari V, Bernardi ML, Cardoso MRI, Barcellos DESN. Virulence profiles of enterotoxigenic Escherichia coli isolated from piglets with post-weaning diarrhea and classification according to fecal consistency. Pesq Vet Bras. 2016; 36:253–257.
Article24. Staden R, Beal KF, Bonfield JK. The Staden package, 1998. Methods Mol Biol. 2000; 132:115–130.
Article25. Vidotto MC, de Lima NC, Fritzen JT, de Freitas JC, Venâncio MJ, Ono MA. Frequency of virulence genes in Escherichia coli strains isolated from piglets with diarrhea in the North Parana State, Brazil. Braz J Microbiol. 2009; 40:199–204.
Article26. Vu Khac H, Holoda E, Pilipcinec E, Blanco M, Blanco JE, Mora A, Dahbi G, López C, González EA, Blanco J. Serotypes, virulence genes, and PFGE profiles of Escherichia coli isolated from pigs with postweaning diarrhoea in Slovakia. BMC Vet Res. 2006; 2:10.27. Wittig W, Klie H, Gallien P, Lehmann S, Timm M, Tschäpe H. Prevalence of the fimbrial antigens F18 and K88 and of enterotoxins and verotoxins among Escherichia coli isolated from weaned pigs. Zentralbl Bakteriol. 1995; 283:95–104.
Article28. Zhang W, Zhao M, Ruesch L, Omot A, Francis D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet Microbiol. 2007; 123:145–152.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Usefulness of Polymerase Chain Reaction for the Diagnosis of Pyosalpinx Caused by Tuberculosis
- Rapid Diagnosis of Duchenne Muscular Dystrophy DMD by Multiplex Polymerase Chain Reaction PCR using Uncultured Amniocytes
- Detection of Mycobacterium tuberculosis DNA using the Polymerase Chain Reaction in Paraffin - embedded Biopsy Specimens of Skin tuberculosis
- Identification of the types of human papillomavirus in condylomata acuminata using polymerase chain reaction
- Polymerase Chain Reaction and Heteroduplex Analysis Based Detection of Clonal T Cell Receptor Gamma Gene Rearrangements in Paraffin-embedded Tissues of Cutaneous T Cell Proliferative Diseases