J Korean Soc Transplant.
2017 Dec;31(4):170-176. 10.4285/jkstn.2017.31.4.170.
Investigation and Standardization on Current Practice of Renal Transplant Pathology in Korea
- Affiliations
-
- 1Department of Pathology, St. Vincent's Hospital, The Catholic University of Korea College of Medicine, Suwon, Korea.
- 2Cancer Research Institute, Chungnam National University School of Medicine, Daejeon, Korea.
- 3National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 4Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. mdyjchoi@catholic.ac.kr
- KMID: 2401567
- DOI: http://doi.org/10.4285/jkstn.2017.31.4.170
Abstract
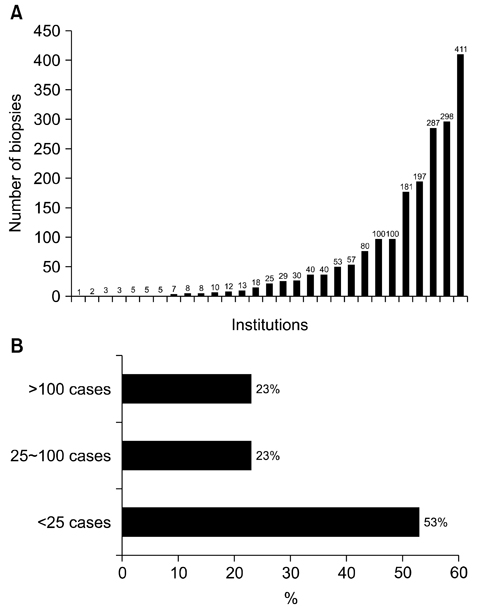
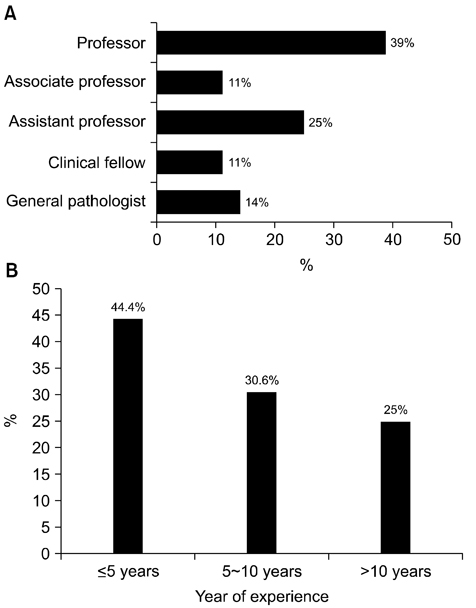
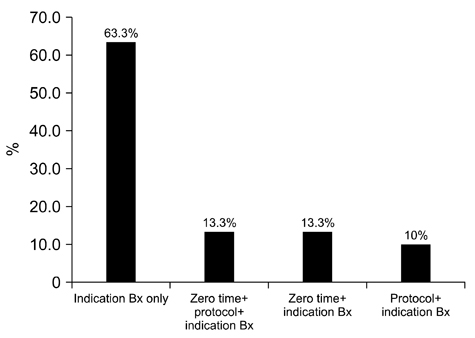
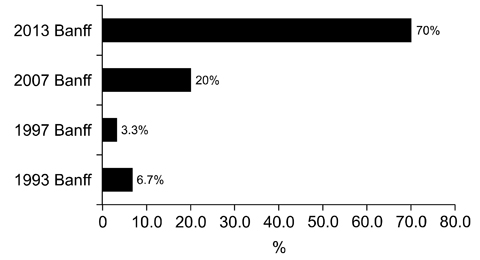
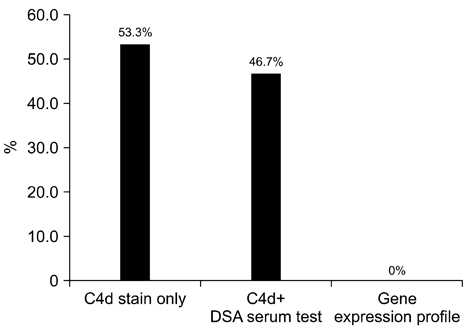
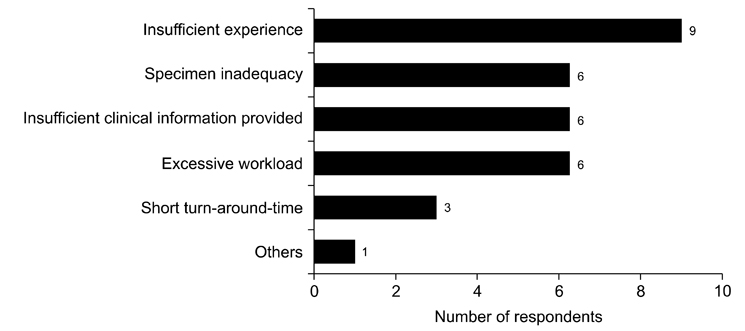
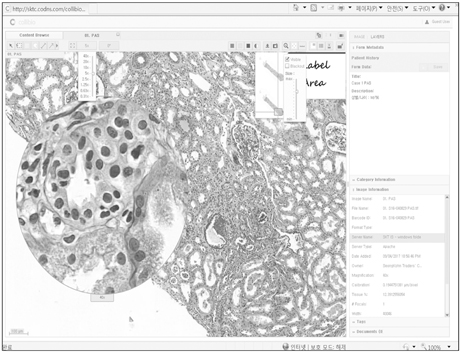
- We need to establish an informative guideline to increase inter-institutional and inter-observer reproducibility of renal transplant diagnosis, and to improve the diagnostic ability of pathologists in Korea. A first nation-wide survey for renal transplant pathology was conducted by Renal Pathology Study Group of the Korean Society of Pathologists in 2016, to provide the continued excellence in the transplantation pathology laboratory, and to improve the diagnostic ability for the best treatment of transplant patients. This survey revealed the significant variations in scale, work load and biopsy indications for the renal transplant pathology in various institutions in Korea. The Banff classification were used by all institutions for the diagnosis of renal transplant pathology, but different formats were used: most institutions (70%) used the "2013 Banff classification" while the others were using "2007 Banff classification" (20%) or even older formats. In daily diagnostic practice of the renal allografts, difficulties that pathologists encounter were quite diverse due to different environments they work in. Most respondents agreed that standardized diagnostic practice guidelines, regular education on renal transplant pathology and convenient ways of consultation are further needed. We are currently working toward the enhancement of the expertise of renal pathologists and to increase inter-institutional and inter-observer reproducibility by 1) development of a set of virtual slides of renal allograft biopsies for the training, 2) validation and gathering expert's consensus on the core variables of rejection diagnosis by using virtual slides, and 3) continued education by the developed virtual slide atlas.
Keyword
MeSH Terms
Figure
Reference
-
1. Broecker V, Mengel M. The significance of histological diagnosis in renal allograft biopsies in 2014. Transpl Int. 2015; 28:136–143.
Article2. Walker PD, Cavallo T, Bonsib SM. Ad Hoc Committee on Renal Biopsy Guidelines of the Renal Pathology Society. Practice guidelines for the renal biopsy. Mod Pathol. 2004; 17:1555–1563.
Article3. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014; 14:272–283.
Article4. Furness PN, Taub N. Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001; 60:1998–2012.
Article5. Roberts I. The U.K. National Renal Pathology External Quality Assessment Scheme [Internet]. Leicester: the University of Leicester;2008. cited 2017 Dec 2. Available from: https://www.le.ac.uk/users/pnf1/eqa/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of renal transplant rejection: Banff classification and beyond
- Recent innovations in renal replacement technology and potential applications to transplantation and dialysis patients: a review of current methods
- The first hair transplant assessment scale: parameters for good hair transplantation
- Recent Advancement in Renal Transplantation
- Surgical experience of transplant renal artery stenosis