J Dent Anesth Pain Med.
2017 Dec;17(4):241-251. 10.17245/jdapm.2017.17.4.241.
Characteristics of electroencephalogram signatures in sedated patients induced by various anesthetic agents
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. byungmoonchoi7@gmail.com
- KMID: 2399794
- DOI: http://doi.org/10.17245/jdapm.2017.17.4.241
Abstract
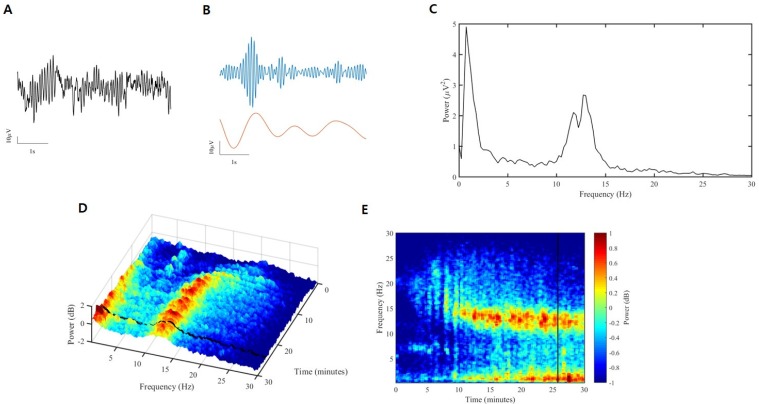
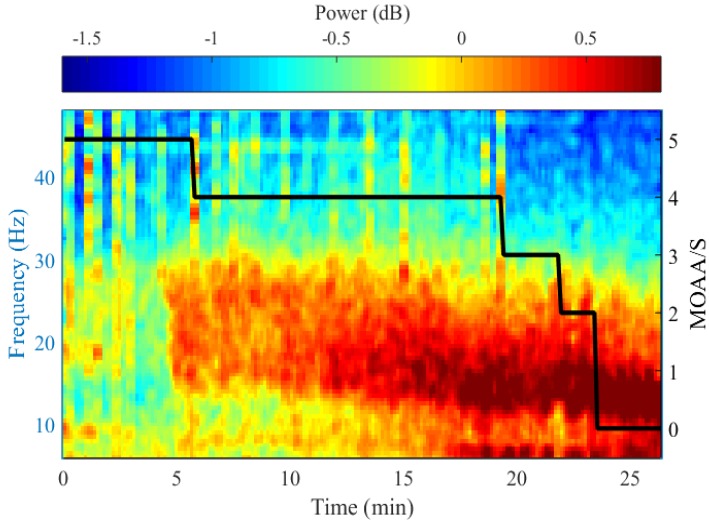
- Devices that monitor the depth of hypnosis based on the electroencephalogram (EEG) have long been commercialized, and clinicians use these to titrate the dosage of hypnotic agents. However, these have not yet been accepted as standard monitoring devices for anesthesiology. The primary reason is that the use of these monitoring devices does not completely prevent awareness during surgery, and the development of these devices has not taken into account the neurophysiological mechanisms of hypnotic agents, thus making it possible to show different levels of unconsciousness in the same brain status. An alternative is to monitor EEGs that are not signal processed with numerical values presented by these monitoring devices. Several studies have reported that power spectral analysis alone can distinguish the effects of different hypnotic agents on consciousness changes. This paper introduces the basic concept of power spectral analysis and introduces the EEG characteristics of various hypnotic agents that are used in sedation.
MeSH Terms
Figure
Reference
-
1. Brazier MA. The Effect of Drugs on the Electroencephalogram of Man. Clin Pharmacol Ther. 1964; 5:102–116. PMID: 14107115.
Article2. Millett D. Hans Berger: from psychic energy to the EEG. Perspect Biol Med. 2001; 44:522–542. PMID: 11600799.
Article3. Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KF, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013; 110:E1142–E1151. PMID: 23487781.
Article4. Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010; 363:2638–2650. PMID: 21190458.
Article5. Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci U S A. 2007; 104:16032–16037. PMID: 17938125.
Article6. Bevan JC, Veall GR, Macnab AJ, Ries CR, Marsland C. Midazolam premedication delays recovery after propofol without modifying involuntary movements. Anesth Analg. 1997; 85:50–54. PMID: 9212121.
Article7. Williams ST, Conte MM, Goldfine AM, Noirhomme Q, Gosseries O, Thonnard M, et al. Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury. Elife. 2013; 2:e01157. PMID: 24252875.
Article8. McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci. 2008; 28:13488–13504. PMID: 19074022.
Article9. Cote CJ, Goudsouzian NG, Liu LM, Dedrick DF, Rosow CE. The dose response of intravenous thiopental for the induction of general anesthesia in unpremedicated children. Anesthesiology. 1981; 55:703–705. PMID: 7305061.10. Gray AT, Krejci ST, Larson MD. Neuromuscular blocking drugs do not alter the pupillary light reflex of anesthetized humans. Arch Neurol. 1997; 54:579–584. PMID: 9152114.
Article11. Feshchenko VA, Veselis RA, Reinsel RA. Propofol-induced alpha rhythm. Neuropsychobiology. 2004; 50:257–266. PMID: 15365226.
Article12. Tinker JH, Sharbrough FW, Michenfelder JD. Anterior shift of the dominant EEG rhytham during anesthesia in the Java monkey: correlation with anesthetic potency. Anesthesiology. 1977; 46:252–259. PMID: 402870.13. Clark DL, Rosner BS. Neurophysiologic effects of general anesthetics. I. The electroencephalogram and sensory evoked responses in man. Anesthesiology. 1973; 38:564–582. PMID: 4145825.14. Hemmings HC Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005; 26:503–510. PMID: 16126282.
Article15. Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABA(A) receptors. J Neurosci. 1999; 19:10635–10646. PMID: 10594047.16. Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures. Anesthesiology. 2015; 123:937–960. PMID: 26275092.17. Akeju O, Pavone KJ, Westover MB, Vazquez R, Prerau MJ, Harrell PG, et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014; 121:978–989. PMID: 25187999.
Article18. Tsukagoshi E, Kawaguchi M, Shinomiya T, Yoshikawa M, Kawano T, Okubo M, et al. Diazepam enhances production of diazepam-binding inhibitor (DBI), a negative saliva secretion regulator, localized in rat salivary gland. J Pharmacol Sci. 2011; 115:221–229. PMID: 21282931.
Article19. Ostuni MA, Issop L, Peranzi G, Walker F, Fasseu M, Elbim C, et al. Overexpression of translocator protein in inflammatory bowel disease: potential diagnostic and treatment value. Inflamm Bowel Dis. 2010; 16:1476–1487. PMID: 20222126.
Article20. Wojna V, Guerrero L, Guzman J, Cotto M. Effect of flumazenil on electroencephalographic patterns induced by midazolam. P R Health Sci J. 2000; 19:353–356. PMID: 11293887.21. Feshchenko VA, Veselis RA, Reinsel RA. Comparison of the EEG effects of midazolam, thiopental, and propofol: the role of underlying oscillatory systems. Neuropsychobiology. 1997; 35:211–220. PMID: 9246224.
Article22. Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011; 34:601–628. PMID: 21513454.
Article24. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995; 52:998–1007. PMID: 7492260.
Article25. Seamans J. Losing inhibition with ketamine. Nat Chem Biol. 2008; 4:91–93. PMID: 18202677.
Article26. Cavazzuti M, Porro CA, Biral GP, Benassi C, Barbieri GC. Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab. 1987; 7:806–811. PMID: 3121648.
Article27. Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol. 1997; 7:25–38. PMID: 9088882.
Article28. Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth. 1993; 71:447–449. PMID: 8104450.
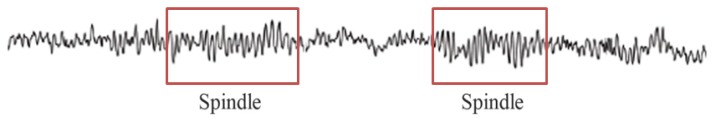
Article29. Nacif-Coelho C, Correa-Sales C, Chang LL, Maze M. Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology. 1994; 81:1527–1534. PMID: 7992922.30. Huupponen E, Maksimow A, Lapinlampi P, Sarkela M, Saastamoinen A, Snapir A, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008; 52:289–294. PMID: 18005372.
Article31. Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998; 18:4705–4721. PMID: 9614245.
Article32. Morairty S, Rainnie D, McCarley R, Greene R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004; 123:451–457. PMID: 14698752.
Article33. Faulconer A, Pender JW, Bickford RG. The influence of partial pressure of nitrous oxide on the depth of anesthesia and the electro-encephalogram in man. Anesthesiology. 1949; 10:601–609. PMID: 18147751.
Article34. Foster BL, Liley DT. Nitrous oxide paradoxically modulates slow electroencephalogram oscillations: implications for anesthesia monitoring. Anesth Analg. 2011; 113:758–765. PMID: 21788312.35. Yamamura T, Fukuda M, Takeya H, Goto Y, Furukawa K. Fast oscillatory EEG activity induced by analgesic concentrations of nitrous oxide in man. Anesth Analg. 1981; 60:283–288. PMID: 7194592.
Article36. Avramov MN, Shingu K, Mori K. Progressive changes in electroencephalographic responses to nitrous oxide in humans: a possible acute drug tolerance. Anesth Analg. 1990; 70:369–374. PMID: 2316879.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Population Pharmacokinetic and Pharmacodynamic Modeling in Beagle Dogs Sedated by Propofol Microemulsion
- Intraoperative nociception monitoring
- Anesthetic Experience with a Case of Bilateral Adrenalectomy for Pheochcomocytoma
- The dual role of transforming growth factor-beta signatures in human B viral multistep hepatocarcinogenesis: early and late responsive genes
- Safety of Sedated Therapeutic Endoscopic Retrograde Cholangiopancreatography in Patients Older than 70 Years Old