Korean J Gastroenterol.
2017 Nov;70(5):223-231. 10.4166/kjg.2017.70.5.223.
Development of Metachronous Tumors after Endoscopic Resection for Gastric Neoplasm according to the Baseline Tumor Grade at a Health Checkup Center
- Affiliations
-
- 1Department of Internal Medicine, Healthcare Research Institute, Healthcare System Gangnam Center, Seoul National University Hospital, Seoul, Korea. medjsj7@hanmail.net
- 2Department of Internal Medicine, Sheikh Khalifa Specialty Hospital, Ras AlKhaimah, UAE.
- 3Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2398868
- DOI: http://doi.org/10.4166/kjg.2017.70.5.223
Abstract
- BACKGROUND/AIMS
Endoscopic resection (ER) procedure has been performed widely to treat gastric neoplasms. Here, we compared the long-term prognosis based on the clinical features of three types of recurred gastric neoplasms after ER, including low-grade dysplasia (LGD), high-grade dysplasia (HGD), and early gastric carcinoma (EGC).
METHODS
Between 2003 and 2014, subjects who were diagnosed with gastric neoplasm during screening endoscopy were included. The baseline clinicopathologic and tumor recurrence were analyzed.
RESULTS
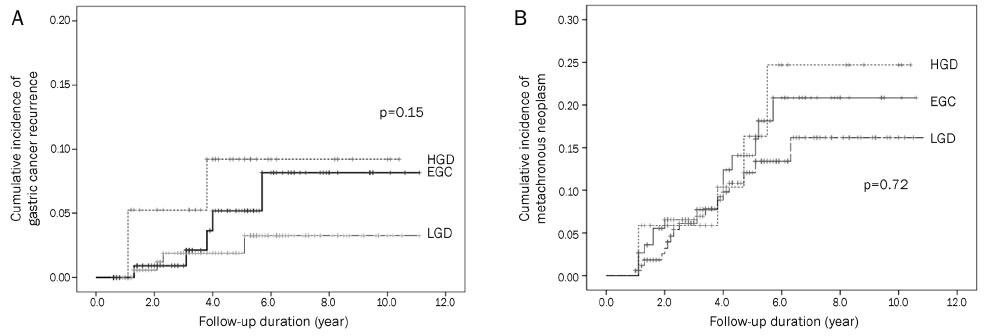
Of the 316 patients enrolled, 170 patients (53.8%) were categorized into the LGD group, 34 patients (10.8%) into the HGD group, and 112 patients (35.4%) into the EGC group. The median follow-up duration was 4.2 years. Among the total, 14 patients experienced a development of metachronous gastric cancer; 4 patients (2.3%) in the LGD group, 3 patients (8.3%) in the HGD group, and 7 patients (6.1%) in the EGC group. Metachronous gastric neoplasm had developed in 17 LGD patients (10.0%), 5 HGD patients (14.7%), and 14 EGC patients (12.5%). There was no significant difference in the incidence of metachronous gastric cancer and neoplasm among the three groups (p=0.15 and p=0.72, respectively).
CONCLUSIONS
We identified that the incidence rates of gastric neoplasm and cancer after endoscopic treatment were not significantly different between the LGD, HGD, and EGC groups.
Keyword
MeSH Terms
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.2. Japanese Gastric Cancer Association Registration Committee. Maruyama K, Kaminishi M, et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006; 9:51–66.3. Orlowska J, Jarosz D, Pachlewski J, Butruk E. Malignant transformation of benign epithelial gastric polyps. Am J Gastroenterol. 1995; 90:2152–2159.4. Fujiwara Y, Arakawa T, Fukuda T, et al. Diagnosis of borderline adenomas of the stomach by endoscopic mucosal resection. Endoscopy. 1996; 28:425–430.5. Kato M, Nishida T, Tsutsui S, et al. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD study group. J Gastroenterol. 2011; 46:325–331.6. Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004; 36:390–396.7. Arima N, Adachi K, Katsube T, et al. Predictive factors for metachronous recurrence of early gastric cancer after endoscopic treatment. J Clin Gastroenterol. 1999; 29:44–47.8. Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013; 62:1425–1432.9. Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005; 37:990–993.10. Lim JH, Kim SG, Choi J, Im JP, Kim JS, Jung HC. Risk factors for synchronous or metachronous tumor development after endoscopic resection of gastric neoplasms. Gastric Cancer. 2015; 18:817–823.11. Seo JH, Park JC, Kim YJ, Shin SK, Lee YC, Lee SK. Undifferentiated histology after endoscopic resection may predict synchronous and metachronous occurrence of early gastric cancer. Digestion. 2010; 81:35–42.12. Maehata Y, Nakamura S, Fujisawa K, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012; 75:39–46.13. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000; 47:251–255.14. Srivastava A, Lauwers GY. Gastric epithelial dysplasia: the Western perspective. Dig Liver Dis. 2008; 40:641–649.15. Lee JY, Choi IJ, Cho SJ, et al. Routine follow-up biopsies after complete endoscopic resection for early gastric cancer may be unnecessary. J Gastric Cancer. 2012; 12:88–98.16. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969; 3:87–97.17. Liu Y, Uemura N, Xiao SD, Tytgat GN, Kate FJ. Agreement between endoscopic and histological gastric atrophy scores. J Gastroenterol. 2005; 40:123–127.18. Yoon SB, Park JM, Lim CH, et al. Incidence of gastric cancer after endoscopic resection of gastric adenoma. Gastrointest Endosc. 2016; 83:1176–1183.19. Yoon H, Kim N, Shin CM, et al. Risk factors for metachronous gastric neoplasms in patients who underwent endoscopic resection of a gastric neoplasm. Gut Liver. 2016; 10:228–236.20. Jung S, Park CH, Kim EH, et al. Preventing metachronous gastric lesions after endoscopic submucosal dissection through Helicobacter pylori eradication. J Gastroenterol Hepatol. 2015; 30:75–81.21. Choi J, Kim SG, Yoon H, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014; 12:793–800.e1.22. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an openlabel, randomised controlled trial. Lancet. 2008; 372:392–397.23. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014; 29:1371–1386.24. Lee SY. New guidelines for Helicobacter pylori treatment: comparisons between Korea and Japan. Korean J Gastroenterol. 2014; 63:151–157.25. Jung DH, Kim JH, Lee YC, et al. Helicobacter pylori eradication reduces the metachronous recurrence of gastric neoplasms by attenuating the precancerous process. J Gastric Cancer. 2015; 15:246–255.26. Papadimitrakopoulou VA, Shin DM, Hong WK. Molecular and cellular biomarkers for field cancerization and multistep process in head and neck tumorigenesis. Cancer Metastasis Rev. 1996; 15:53–76.27. de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008; 134:945–952.28. Park DI, Rhee PL, Kim JE, et al. Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy. 2001; 33:501–506.29. Lage J, Uedo N, Dinis-Ribeiro M, Yao K. Surveillance of patients with gastric precancerous conditions. Best Pract Res Clin Gastroenterol. 2016; 30:913–922.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Helicobacter pylori Eradication on the Metachronous Neoplasm after Endoscopic Resection for Gastric Dysplasia
- Utility of Surgical Resection in the Management of Metachronous Krukenberg's Tumors of Gastric Origin
- Effectiveness of Helicobacter pylori Eradication before Endoscopic Resection
- Endoscopic Treatment for Gastric Subepithelial Tumor
- Recurrence after endoscopic resection of small rectal neuroendocrine tumors: a retrospective cohort study