Korean J Physiol Pharmacol.
2018 Jan;22(1):81-89. 10.4196/kjpp.2018.22.1.81.
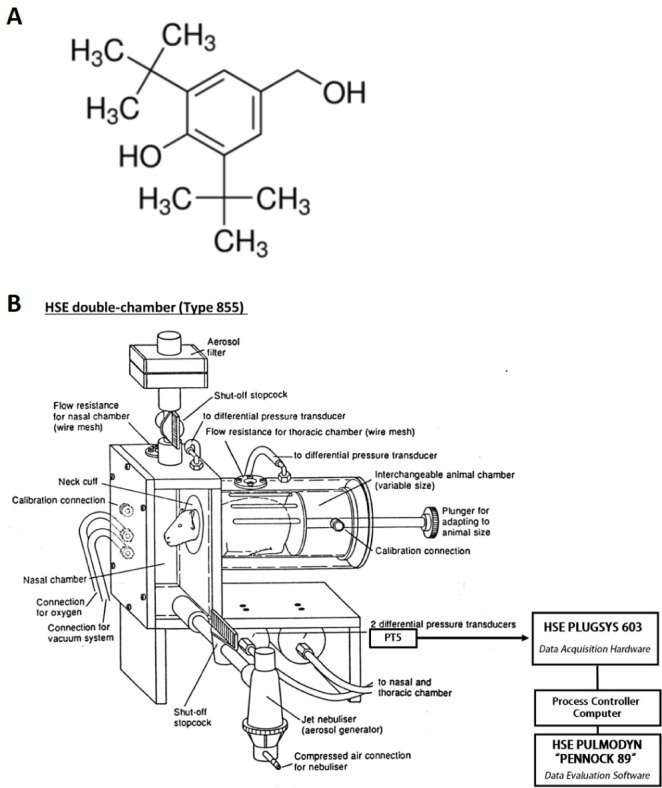
Inhibitory effects of 2,6-di-tert-butyl-4-hydroxymethylphenol on asthmatic responses to ovalbumin challenge in conscious guinea pigs
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 06974, Korea. jylee98@cau.ac.kr
- KMID: 2398558
- DOI: http://doi.org/10.4196/kjpp.2018.22.1.81
Abstract
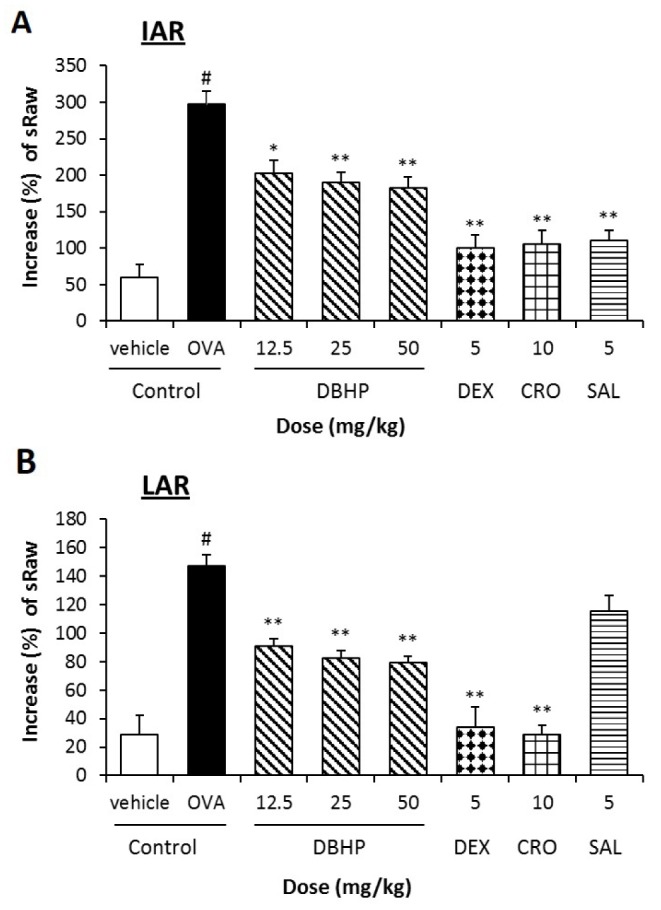
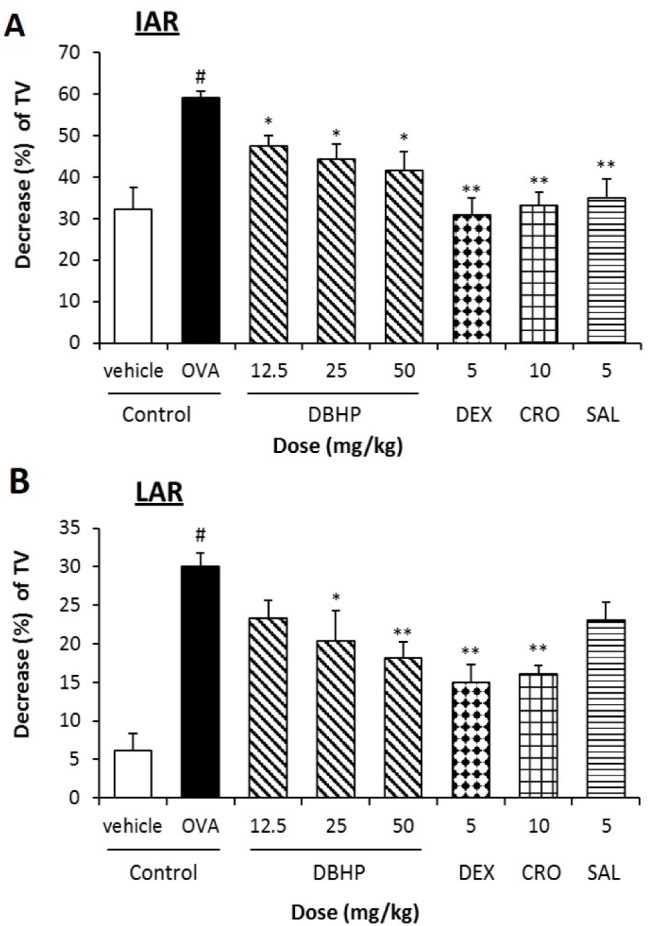
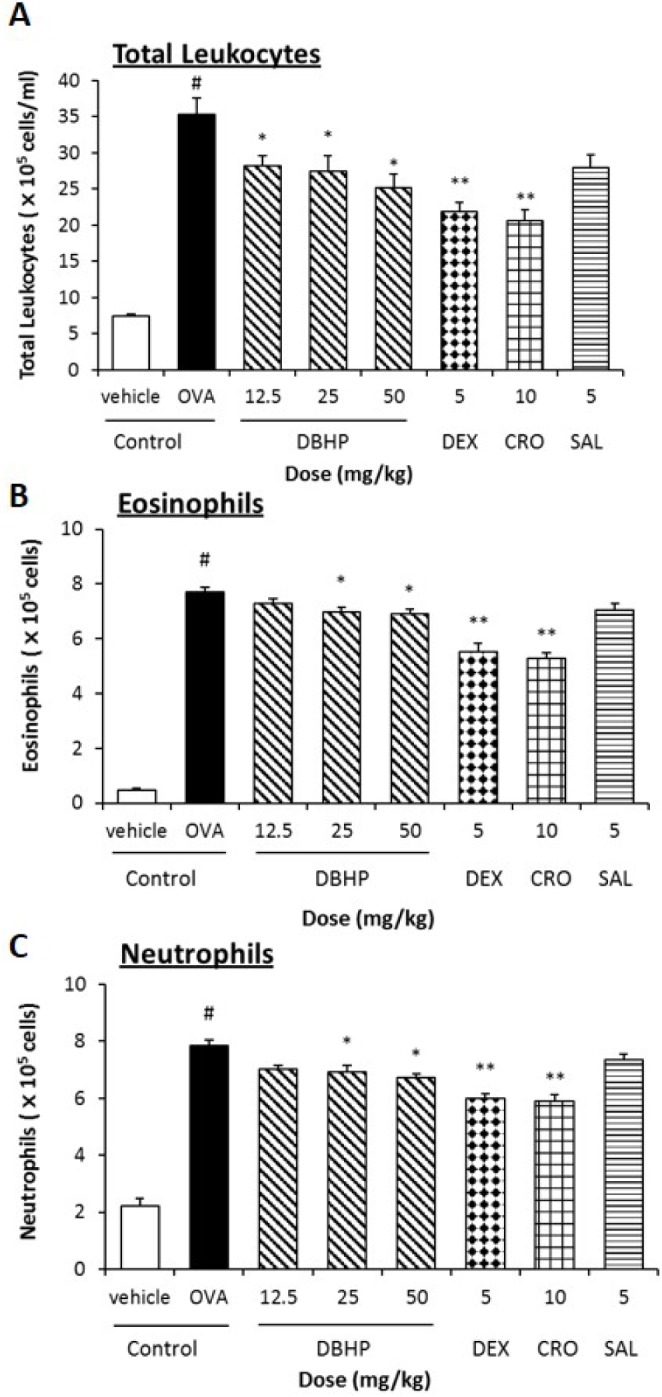
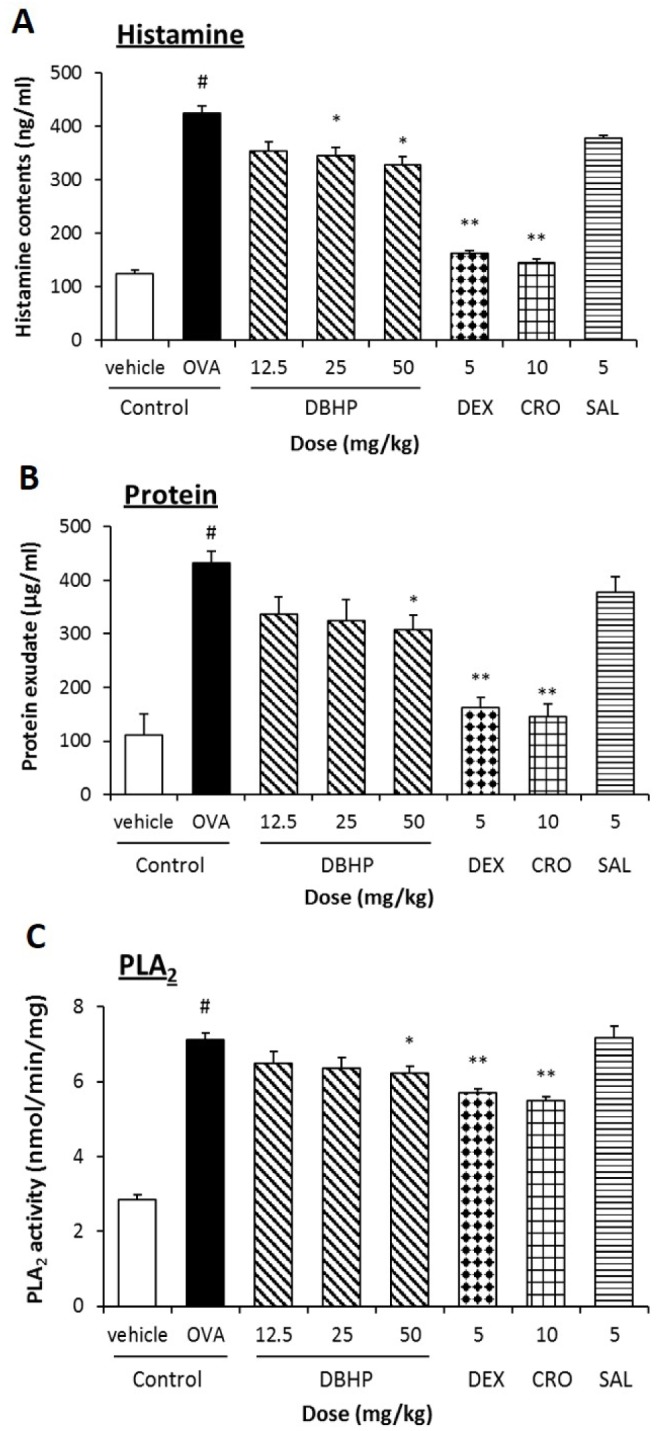
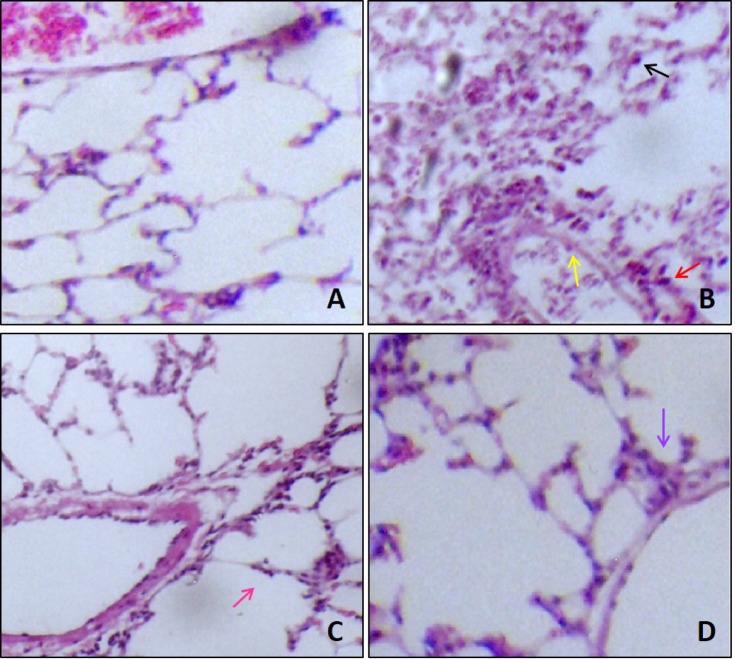
- This study evaluated the anti-asthmatic activities of 2,6-di-tert-butyl-4-hydroxymethylphenol (DBHP) that is a potent phenolic antioxidant in edible vegetable oil. The effects of DBHP on bronchial asthma were evaluated by determining the specific airway resistance (sRaw) and tidal volume (TV) during the immediate asthmatic response (IAR) and the late-phase asthmatic response (LAR) in guinea pigs with aerosolized ovalbumin-induced asthma. Recruitment of leukocytes and the levels of biochemical inflammatory mediators were determined in the bronchoalveolar lavage fluids (BALFs), and histopathological surveys performed in lung tissues. DBHP significantly inhibited the increased sRaw and improved the decreased TV on IAR and LAR, and also inhibited recruitment of eosinophils and neutrophils into the lung, and release of biochemical inflammatory mediators such as histamine and phospholipase Aâ‚‚ from these infiltrated leukocytes, and improved pathological changes. However, anti-asthmatic activities of DBHP at oral doses of 12.5 to 50 mg/kg was less than those of dexamethasone (5 mg/kg, p.o.) and cromoglycate (10 mg/kg, p.o.), but more potent or similar to that of salbutamol (5 mg/kg, p.o.). These results in the present study suggest that anti-asthmatic effects of DBHP in the guinea pigs model of OVA-induced asthmatic responses principally are mediated by inhibiting the recruitments of the leukocytes and the release of biochemical inflammatory mediators from these infiltrated leukocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Leick EA, Reis FG, Honorio-Neves FA, Almeida-Reis R, Prado CM, Martins MA, Tibério IF. Effects of repeated stress on distal airway inflammation, remodeling and mechanics in an animal model of chronic airway inflammation. Neuroimmunomodulation. 2012; 19:1–9. PMID: 22067616.
Article2. Hyung KE, Kim SJ, Jang YW, Lee DK, Hyun KH, Moon BS, Kim B, Ahn H, Park SY, Sohn UD, Park ES, Hwang KW. Therapeutic effects of orally administered CJLP55 for atopic dermatitis via the regulation of immune response. Korean J Physiol Pharmacol. 2017; 21:335–343. PMID: 28461776.
Article3. Matsumoto T, Ashida Y, Tsukuda R. Pharmacological modulation of immediate and late airway response and leukocyte infiltration in the guinea pig. J Pharmacol Exp Ther. 1994; 269:1236–1244. PMID: 8014867.4. Sanjar S, Aoki S, Kristersson A, Smith D, Morley J. Antigen challenge induces pulmonary airway eosinophil accumulation and airway hyperreactivity in sensitized guinea-pigs: the effect of anti-asthma drugs. Br J Pharmacol. 1990; 99:679–686. PMID: 2361168.
Article5. Hanania NA. Targeting airway inflammation in asthma: current and future therapies. Chest. 2008; 133:989–998. PMID: 18398119.6. Saji J, Yamamoto T, Arai M, Mineshita M, Miyazawa T. Efficacy of long-term omalizumab therapy in patients with severe asthma. Respir Investig. 2017; 55:114–120.
Article7. Alves MF, da Fonsec DV, de Melo SAL, Scotti MT, Scotti L, Dos Santos SG, de Fátima Formiga Melo Diniz M. New therapeutic targets and drugs for the treatment of asthma. Mini Rev Med Chem. 2017; 100:317.
Article8. Wex E, Thaler E, Blum S, Lamb D. A novel model of IgE-mediated passive pulmonary anaphylaxis in rats. PLoS One. 2014; 9:e116166. PMID: 25541997.
Article10. Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev. 2010; (5):CD005535. PMID: 20464739.
Article11. Jung CH, Lee JY, Cho CH, Kim CJ. Anti-asthmatic action of quercetin and rutin in conscious guinea-pigs challenged with aerosolized ovalbumin. Arch Pharm Res. 2007; 30:1599–1607. PMID: 18254248.
Article12. Moon H, Choi HH, Lee JY, Moon HJ, Sim SS, Kim CJ. Quercetin inhalation inhibits the asthmatic responses by exposure to aerosolized-ovalbumin in conscious guinea-pigs. Arch Pharm Res. 2008; 31:771–778. PMID: 18563360.
Article13. Suh SJ, Kwak CH, Chung TW, Park SJ, Cheeeei M, Park SS, Seo CS, Son JK, Chang YC, Park YG, Lee YC, Chang HW, Kim CH. Pimaric acid from Aralia cordata has an inhibitory effect on TNF-α-induced MMP-9 production and HASMC migration via down-regulated NF-κB and AP-1. Chem Biol Interact. 2012; 199:112–119. PMID: 22705379.14. Pennock BE, Cox CP, Rogers RM, Cain WA, Wells JH. A noninvasive technique for measurement of changes in specific airway resistance. J Appl Physiol Respir Environ Exerc Physiol. 1979; 46:399–406. PMID: 422457.
Article15. Comas-Basté O, Latorre-Moratalla ML, Bernacchia R, Veciana-Nogués MT, Vidal-Carou MC. New approach for the diagnosis of histamine intolerance based on the determination of histamine and methylhistamine in urine. J Pharm Biomed Anal. 2017; 145:379–385. PMID: 28715791.
Article16. Song HS, Park SH, Ko MS, Jeong JM, Sohn UD, Sim SS. Morinda citrifolia inhibits both cytosolic Ca2+-dependent phospholipase A2 and secretory Ca2+-dependent phospholipase A2. Korean J Physiol Pharmacol. 2010; 14:163–167. PMID: 20631889.17. Saeed A, Khan SU, Mahesar PA, Channar PA, Shabir G, Iqbal J. Substituted (E)-2-(2-benzylidenehydrazinyl)-4-methylthiazole-5-carboxylates as dual inhibitors of 15-lipoxygenase & carbonic anhydrase II: synthesis, biochemical evaluation and docking studies. Biochem Biophys Res Commun. 2017; 482:176–181. PMID: 27836541.18. Lee JY, Kim JM, Kim CJ. Flavones derived from nature attenuate the immediate and late-phase asthmatic responses to aerosolized-ovalbumin exposure in conscious guinea pigs. Inflamm Res. 2014; 63:53–60. PMID: 24142298.
Article19. Antwi AO, Obiri DD, Osafo N. Stigmasterol modulates allergic airway inflammation in guinea pig model of ovalbumin-induced asthma. Mediators Inflamm. 2017; 2017:2953930. PMID: 28555089.
Article20. Hoymann HG. Lung function measurements in rodents in safety pharmacology studies. Front Pharmacol. 2012; 3:156. PMID: 22973226.
Article21. Chong BT, Agrawal DK, Romero FA, Townley RG. Measurement of bronchoconstriction using whole-body plethysmograph: comparison of freely moving versus restrained guinea pigs. J Pharmacol Toxicol Methods. 1998; 39:163–168. PMID: 9741391.
Article22. Jang TY, Jung AY, Kyung TS, Kim DY, Hwang JH, Kim YH. Anti-allergic effect of luteolin in mice with allergic asthma and rhinitis. Cent Eur J Immunol. 2017; 42:24–29. PMID: 28680328.
Article23. Ujino M, Sugimoto N, Koizumi Y, Ro S, Kojima Y, Asae KH, Yamashita N, Ohta K, Nagase H. Leukotriene receptor antagonist attenuated airway inflammation and hyperresponsiveness in a double-stranded RNA-induced asthma exacerbation model. Allergol Int. 2017; 66S:S21–S26. PMID: 28647381.
Article24. Yan S, Ci X, Chen N, Chen C, Li X, Chu X, Li J, Deng X. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm Res. 2011; 60:589–596. PMID: 21279416.
Article25. Jang YW, Lee JY, Kim CJ. Anti-asthmatic activity of phenolic compounds from the roots of Gastrodia elata Bl. Int Immunopharmacol. 2010; 10:147–154. PMID: 19874915.26. Xavier CV, da S Setúbal S, Lacouth-Silva F, Pontes AS, Nery NM, de Castro OB, Fernandes CFC, Soares AM, Fortes-Dias CL, Zuliani JP. Phospholipase A2 inhibitor from Crotalus durissus terrificus rattle-snake: Effects on human peripheral blood mononuclear cells and human neutrophils cells. Int J Biol Macromol. 2017; 105:1117–1125. PMID: 28743568.27. Keapai W, Apichai S, Amornlerdpison D, Lailerd N. Evaluation of fish oil-rich in MUFAs for anti-diabetic and anti-inflammation potential in experimental type 2 diabetic rats. Korean J Physiol Pharmacol. 2016; 20:581–593. PMID: 27847435.
Article28. Lukic A, Ji J, Idborg H, Samuelsson B, Palmberg L, Gabrielsson S, Radmark O. Pulmonary epithelial cancer cells and their exosomes metabolize myeloid cell-derived leukotriene C4 to leukotriene D4. J Lipid Res. 2016; 57:1659–1669. PMID: 27436590.
Article29. Kim HJ, Yoo HY. Hypoxic pulmonary vasoconstriction and vascular contractility in monocrotaline-induced pulmonary arterial hypertensive rats. Korean J Physiol Pharmacol. 2016; 20:641–647. PMID: 27847441.
Article30. Sultan MT, Li HM, Lee YZ, Lim SS, Song DK. Identification of Lys49-PLA2 from crude venom of Crotalus atrox as a human neutrophil-calcium modulating protein. Korean J Physiol Pharmacol. 2016; 20:177–183. PMID: 26937214.31. Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, Park JW, Park EK, Shin HI, Kim SH. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007; 56:210–215. PMID: 17588137.
Article32. Li X, Shen Y, Lu Y, Yang J. Amelioration of bleomycin-induced pulmonary fibrosis of rats by an aldose reductase inhibitor, epalrestat. Korean J Physiol Pharmacol. 2015; 19:401–411. PMID: 26330752.
Article33. Li N, Qiu R, Yang Z, Li J, Chung KF, Zhong N, Zhang Q. Sputum microbiota in severe asthma patients: relationship to eosinophilic inflammation. Respir Med. 2017; 131:192–198. PMID: 28947029.
Article34. Baldissera L Jr, Squebola-Cola DM, Calixto MC, Lima-Barbosa AP, Rennó AL, Anhê GF, Condino-Neto A, De Nucci G, Antunes E. The soluble guanylyl cyclase activator BAY 60-2770 inhibits murine allergic airways inflammation and human eosinophil chemotaxis. Pulm Pharmacol Ther. 2016; 41:86–95. PMID: 27816773.35. Chaiyasit W, McClements DJ, Decker EA. The relationship between the physicochemical properties of antioxidants and their ability to inhibit lipid oxidation in bulk oil and oil-in-water emulsions. J Agric Food Chem. 2005; 53:4982–4988. PMID: 15941345.
Article36. Brekke OL, Shalaby MR, Sundan A, Espevik T, Bjerve KS. Butylated hydroxyanisole specifically inhibits tumor necrosis factor-induced cytotoxicity and growth enhancement. Cytokine. 1992; 4:269–280. PMID: 1515551.
Article37. Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet. 1994; 344:721–724. PMID: 7915779.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The comparison of ovalbumin sensitization by intraperitoneal injection and by inhalation in the development of guinea pig asthma model
- Early Bronchoconstriction After Allergen Challenge of Nonanesthetized Guinea Pigs
- Effects of anesthesia on the electrically-evoked middle latency responses on guinea pigs
- Experimental Dermatitis by Pityrosporum ovale in Guinea Pigs
- Anti-Allergic Effects of Aloe Glycoprotein (NY945) on Ovalbumin-Induced Airway Allergy in the Guinea Pig