Ann Surg Treat Res.
2017 Dec;93(6):322-330. 10.4174/astr.2017.93.6.322.
Comparison of partially-absorbable lightweight mesh with heavyweight mesh for inguinal hernia repair: multicenter randomized study
- Affiliations
-
- 1Department of Surgery, Daehang Hospital, Seoul, Korea.
- 2Department of Surgery, Eulji Medical Center, Eulji University College of Medicine, Seoul, Korea. cutdown@eulji.ac.kr
- KMID: 2396874
- DOI: http://doi.org/10.4174/astr.2017.93.6.322
Abstract
- PURPOSE
Prosthetic mesh is widely used for inguinal hernia repair; however, pain and stiffness can develop. This study was a prospective, multicenter, single-blind, randomized trial to assess postoperative pain and quality of life according to mesh type after inguinal hernia repair.
METHODS
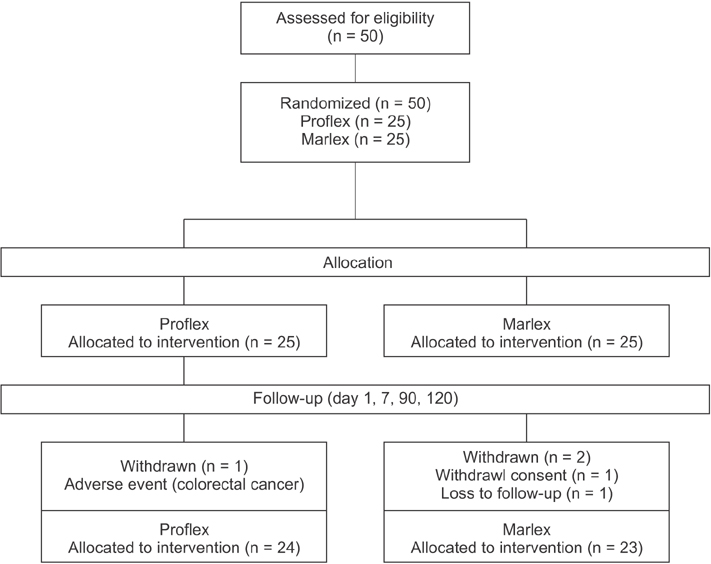
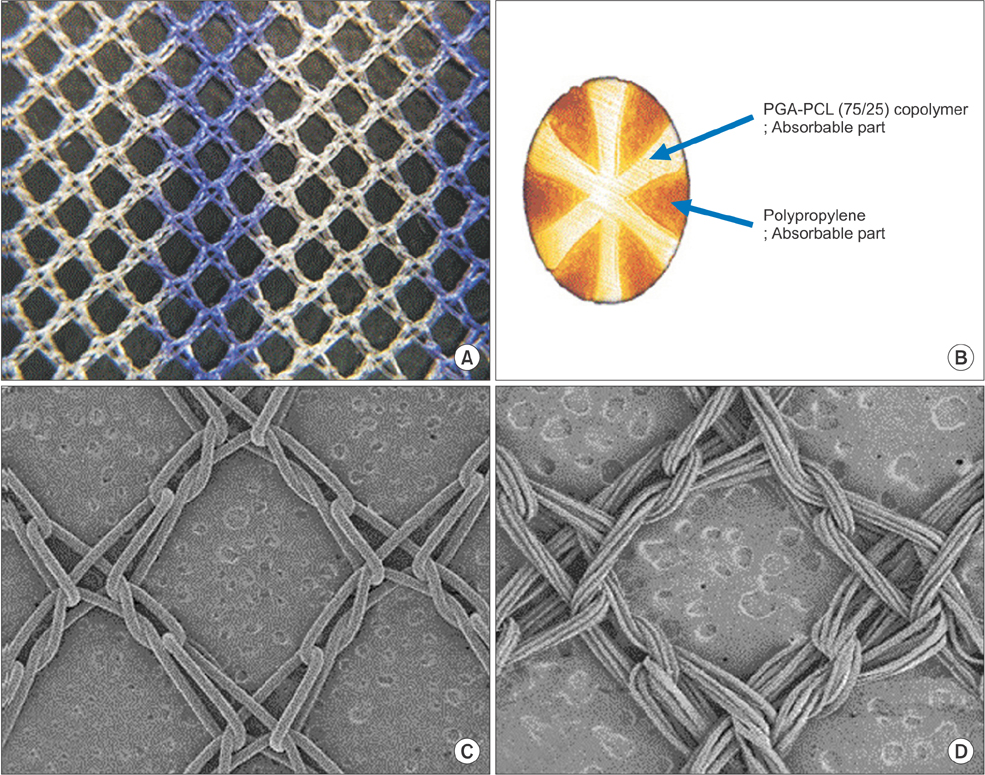
Forty-seven patients who underwent Lichtenstein repair for unilateral inguinal hernia with prosthetic mesh were enrolled and randomly allocated to the partially-absorbable lightweight mesh (LW group, n = 24) or heavyweight mesh group (HW group, n = 23). Data were collected using a visual analogue scale (VAS), Carolinas Comfort Scale (CCS), and Activities Assessment Scale (AAS) at screening and postoperative day 1, 7, 90, and 120; foreign body sensation, sense of stiffness, and sense of pull during activity were also evaluated.
RESULTS
There were no significant differences in patients' demographics and clinical characteristics between groups. The VAS at day 90 was significantly lower in the LW group (0.46 ± 0.78 vs. 0.96 ± 0.82, P = 0.027). The CCS and AAS were significantly lower in the LW group at day 1 (51.33 ± 20.29 vs. 64.65 ± 22.64, P = 0.047 and 39.83 ± 9.88 vs. 46.43 ± 7.82, P = 0.015, respectively). Foreign body sensation was significantly lower in the LW group at day 120 (4.2% vs. 30.4 %, P = 0.023), as was sense of stiffness (P = 0.023). The sense of pull during activity was lower in the LW group at day 90 and 120 (P = 0.012 and P = 0.022, respectively). There was no recurrence or serious complication during follow-up.
CONCLUSION
Partially-absorbable lightweight prosthetic mesh can be used for inguinal hernia repair safely and improve functional outcomes and quality of life after surgery.
Keyword
MeSH Terms
Figure
Reference
-
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003; 83:1045–1051. v–vi.2. Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia. 2009; 13:343–403.3. Baktir A, Dogru O, Girgin M, Aygen E, Kanat BH, Dabak DO, et al. The effects of different prosthetic materials on the formation of collagen types in incisional hernia. Hernia. 2013; 17:249–253.4. Zhu LM, Schuster P, Klinge U. Mesh implants: an overview of crucial mesh parameters. World J Gastrointest Surg. 2015; 7:226–236.5. McCarthy M Jr, Chang CH, Pickard AS, Giobbie-Hurder A, Price DD, Jonasson O, et al. Visual analog scales for assessing surgical pain. J Am Coll Surg. 2005; 201:245–252.6. Heniford BT, Walters AL, Lincourt AE, Novitsky YW, Hope WW, Kercher KW. Comparison of generic versus specific quality-of-life scales for mesh hernia repairs. J Am Coll Surg. 2008; 206:638–644.7. McCarthy M Jr, Jonasson O, Chang CH, Pickard AS, Giobbie-Hurder A, Gibbs J, et al. Assessment of patient functional status after surgery. J Am Coll Surg. 2005; 201:171–178.8. Klinge U, Klosterhalfen B. Modified classification of surgical meshes for hernia repair based on the analyses of 1,000 explanted meshes. Hernia. 2012; 16:251–258.9. Shin D, Lipshultz LI, Goldstein M, Barmé GA, Fuchs EF, Nagler HM, et al. Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction: a preventable cause of obstructive azoospermia. Ann Surg. 2005; 241:553–558.10. Kim M, Oommen B, Ross SW, Lincourt AE, Matthews BD, Heniford BT, et al. The current status of biosynthetic mesh for ventral hernia repair. Surg Technol Int. 2014; 25:114–121.11. Sajid MS, Leaver C, Baig MK, Sains P. Systematic review and meta-analysis of the use of lightweight versus heavyweight mesh in open inguinal hernia repair. Br J Surg. 2012; 99:29–37.12. Li J, Ji Z, Cheng T. Lightweight versus heavyweight in inguinal hernia repair: a meta-analysis. Hernia. 2012; 16:529–539.13. Greca FH, de Paula JB, Biondo-Simoes ML, da Costa FD, da Silva AP, Time S, et al. The influence of differing pore sizes on the biocompatibility of two polypropylene meshes in the repair of abdominal defects. Experimental study in dogs. Hernia. 2001; 5:59–64.14. Greca FH, Souza-Filho ZA, Giovanini A, Rubin MR, Kuenzer RF, Reese FB, et al. The influence of porosity on the integration histology of two polypropylene meshes for the treatment of abdominal wall defects in dogs. Hernia. 2008; 12:45–49.15. O'Dwyer PJ, Kingsnorth AN, Molloy RG, Small PK, Lammers B, Horeyseck G. Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. Br J Surg. 2005; 92:166–170.16. Paajanen H. A single-surgeon randomized trial comparing three composite meshes on chronic pain after Lichtenstein hernia repair in local anesthesia. Hernia. 2007; 11:335–339.17. Aasvang EK, Mohl B, Bay-Nielsen M, Kehlet H. Pain related sexual dysfunction after inguinal herniorrhaphy. Pain. 2006; 122:258–263.18. Burgmans JP, Voorbrood CE, Simmermacher RK, Schouten N, Smakman N, Clevers G, et al. Long-term results of a randomized double-blinded prospective trial of a lightweight (Ultrapro) versus a heavyweight mesh (Prolene) in laparoscopic total extra-peritoneal inguinal hernia repair (TULP-trial). Ann Surg. 2016; 263:862–866.19. Zogbi L, Trindade EN, Trindade MR. Compa rative study of shrinkage, inflammatory response and fibroplasia in heavyweight and lightweight meshes. Hernia. 2013; 17:765–772.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and safety of a novel partially absorbable mesh in totally extraperitoneal hernia repair
- A Comparision of the Mesh Technique in Inguinal Hernia Repair with the Non-mesh Method
- Management of Infected Mesh after Laparoscopic Incisional Hernia Repair
- Outcome of the patients with chronic mesh infection following open inguinal hernia repair
- Inguinal hernia repair with or without mesh in late adolescent males