Yonsei Med J.
2008 Feb;49(1):119-128.
Comparison of Effects of Alendronate and Raloxifene on Lumbar Bone Mineral Density, Bone Turnover, and Lipid Metabolism in Elderly Women with Osteoporosis
- Affiliations
-
- 1Department of Sports Medicine, Keio University School of Medicine, Tokyo, Japan. jiwamoto@sc.itc.keio.ac.jp
- 2Department of Neurology, Mitate Hospital, Fukuoka, Japan.
- 3Department of Orthopaedic Surgery, Keiyu Orthopaedic Hospital, Gunma, Japan.
Abstract
- PURPOSE
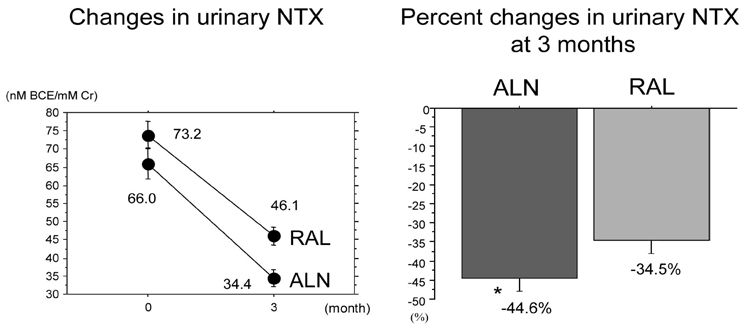
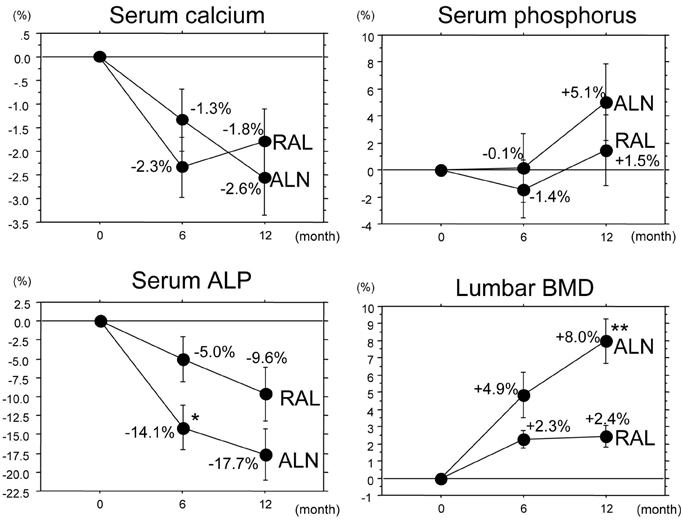
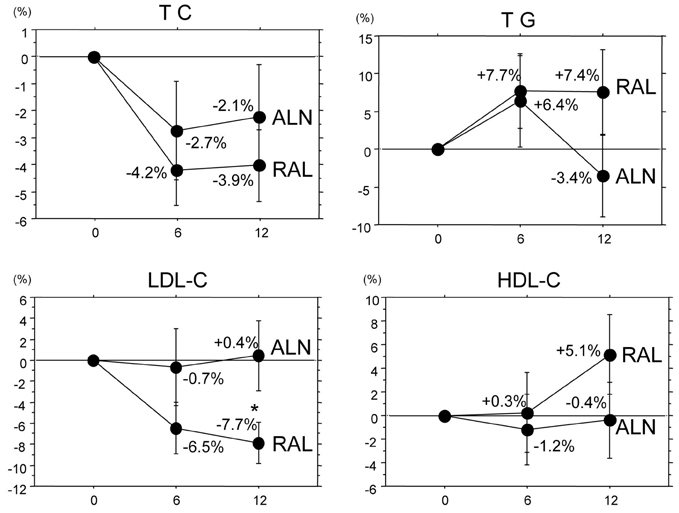
To compare the effects of alendronate and raloxifene on lumbar bone mineral density (BMD), bone turnover, and lipid metabolism in elderly women with osteoporosis. Subjects and Methods: One hundred twenty-two postmenopausal women with osteoporosis (mean age: 69.4 years) were randomly divided into 2 groups of 61 patients: the alendronate group and the raloxifene group. BMD of the lumbar spine, urinary level of cross-linked N-terminal telopeptides of type I collagen (NTX), and serum levels of alkaline phosphatase (ALP), total cholesterol (TC), high and low density lipoprotein cholesterols (LDL-C and HDL-C, respectively), and triglycerides (TG) were measured during the 12-month-treatment period. RESULTS: The trial in 50 patients in the alendronate group and 52 patients in the raloxifene group could be completed. Both alendronate and raloxifene increased lumbar BMD (+8.0% and +2.4% at 12 months, respectively), followed by reductions of urinary NTX level and serum ALP level; however, the effects of alendronate were more pronounced than those of raloxifene. Only raloxifene reduced the serum levels of TC and LDL-C (-3.9% and -7.7% at 12 months, respectively), without any significant effect on the serum HDL-C and TG levels. CONCLUSION: The present study confirmed the efficacy of alendronate greater than raloxifene in increasing lumbar BMD through its effect on marked reduction of the bone turnover more than by raloxifene, and some beneficial effects of raloxifene on lipid metabolism in elderly women with osteoporosis.
MeSH Terms
-
Aged
Alendronate/adverse effects/pharmacology/*therapeutic use
Biological Markers/blood
Bone Density/*drug effects
Calcium/blood
Female
Fractures, Bone/prevention & control
Humans
Lipid Metabolism/*drug effects
Osteoporosis/*drug therapy/*metabolism
Phosphorus/blood
Raloxifene/adverse effects/pharmacology/*therapeutic use
Spine/drug effects
Figure
Reference
-
1. Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. J Am Geriatr Soc. 2000. 48:241–249.
Article2. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003. 32:468–473.
Article3. Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002. 23:570–578.
Article4. Papapoulos SE, Quandt SA, Liberman UA, Hochberg MC, Thompson DE. Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int. 2005. 16:468–474.
Article5. Siris ES, Harris ST, Eastell R, Zanchetta JR, Goemaere S, Diez-Perez A, et al. Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to Evista (CORE) study. J Bone Miner Res. 2005. 20:1514–1524.
Article6. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004. 350:1189–1199.
Article7. Liberman UA, Hochberg MC, Geusens P, Shah A, Lin J, Chattopadhyay A, et al. Hip and non-spine fracture risk reductions differ among antiresorptive agents: Evidence from randomised controlled trials. Int J Clin Pract. 2006. 60:1394–1400.
Article8. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999. 282:637–645.
Article9. Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998. 280:2077–2082.
Article10. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996. 348:1535–1541.
Article11. Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997. 337:1641–1647.
Article12. Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, et al. Diagnostic criteria of primary osteoporosis. J Bone Miner Metab. 1998. 16:139–150.
Article13. Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001. 19:331–337.
Article14. Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, et al. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. Osteoporos Int. 1999. 10:183–192.
Article15. Kushida K, Shiraki M, Nakamura T, Kishimoto H, Morii H, Yamamoto K, et al. The efficacy of alendronate in reducing the risk for vertebral fracture in Japanese patients with osteoporosis: a randomized, double-blind, active-controlled, double-dummy trial. Current Therapeutic Research. 2002. 63(9):606–620.
Article16. Morii H, Ohashi Y, Taketani Y, Fukunaga M, Nakamura T, Itabashi A, et al. Effect of raloxifene on bone mineral density and biochemical markers of bone turnover in Japanese postmenopausal women with osteoporosis: results from a randomized placebo-controlled trial. Osteoporos Int. 2003. 14:793–800.
Article17. Sambrook PN, Geusens P, Ribot C, Solimano JA, Ferrer-Barriendos J, Gaines K, et al. Alendronate produces greater effects than raloxifene on bone density and bone turnover in postmenopausal women with low bone density: results of EFFECT (Efficacy of FOSAMAX versus EVISTA Comparison Trial) International. J Intern Med. 2004. 255:503–511.
Article18. Iwamoto J, Takeda T, Sato Y, Uzawa M. Early changes in urinary cross-linked N-terminal telopeptides of type I collagen level correlate with 1-year response of lumbar bone mineral density to alendronate in postmenopausal Japanese women with osteoporosis. J Bone Miner Metab. 2005. 23:238–242.
Article19. Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994. 79:1693–1700.
Article20. Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res. 1998. 13:1431–1438.
Article21. Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000. 85:3109–3115.
Article22. Rogers MJ, Frith JC, Luckman SP, Coxon FR, Benford HL, Mönkkönen J, et al. Molecular mechanism of action of bisphosphonates. Bone. 1999. 24:5 Suppl. 73S–79S.23. Nakamura T, Liu JL, Morii H, Huang QR, Zhu HM, Qu Y, et al. Effect of raloxifene on clinical fractures in Asian women with postmenopausal osteoporosis. J Bone Miner Metab. 2006. 24:414–418.
Article24. Lufkin EG, Sarkar S, Kulkarni PM, Ciaccia AV, Siddhanti S, Stock J, et al. Antiresorptive treatment of postmenopausal osteoporosis: review of randomized clinical studies and rationale for the Evista alendronate comparison (EVA) trial. Curr Med Res Opin. 2004. 20:351–357.
Article25. Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002. 112:281–289.
Article26. Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002. 87:1586–1592.
Article27. Epstein S. The roles of bone mineral density, bone turnover, and other properties in reducing fracture risk during antiresorptive therapy. Mayo Clin Proc. 2005. 80:379–388.
Article28. Cryer B, Bauer DC. Oral bisphosphonates and upper gastrointestinal tract problems: what is the evidence? Mayo Clin Proc. 2002. 77:1031–1043.
Article29. Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, et al. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J Bone Miner Metab. 2005. 23:97–104.
Article30. Kung AW, Chao HT, Huang KE, Need AG, Taechakraichana N, Loh FH, et al. Efficacy and safety of raloxifene 60 mg/day in postmenopausal Asian women. J Clin Endocrinol Metab. 2003. 88:3130–3136.
Article31. Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004. 96:1751–1761.
Article32. Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006. 355:125–137.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Paper “Comparison of Effects of Alendronate and Raloxifene on Lumbar Bone Mineral Density, Bone Turnover, and Lipid Metabolism in Elderly Women with Osteoporosis†by Iwamoto J, et al. [Yonsei Med J 2008;49(1):119-128]
- Effects of the Combined Therapy with Raloxifene and Low-Dose Intermittent Fluoride for Two Years in Postmenopausal Women with Osteoporosis
- Combination Therapy of Raloxifene and Alendronate for Treatment of Osteoporosis in Elderly Women
- Comparison of Bone Mineral Density of Lumbar Spine in Osteoporotic Patients Treated with Percutaneous Vertebroplasty
- Medical Treatment of Osteoporosis