Yonsei Med J.
2008 Feb;49(1):19-27.
FLIP as an Anti-Cancer Therapeutic Target
- Affiliations
-
- 1Department of Chemistry, School of Natural Sciences, Soongsil University, Seoul, Korea. jinkukyang@ssu.ac.kr
Abstract
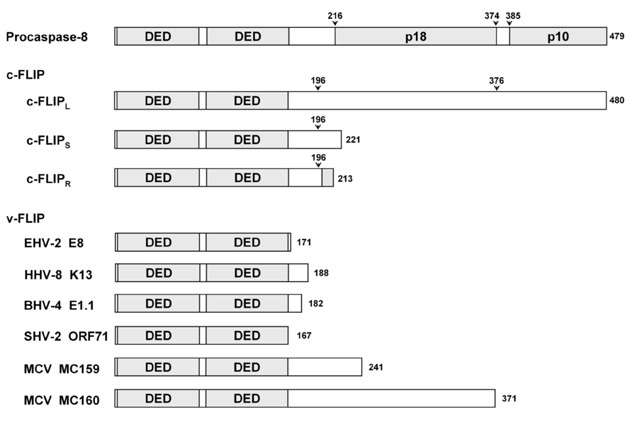
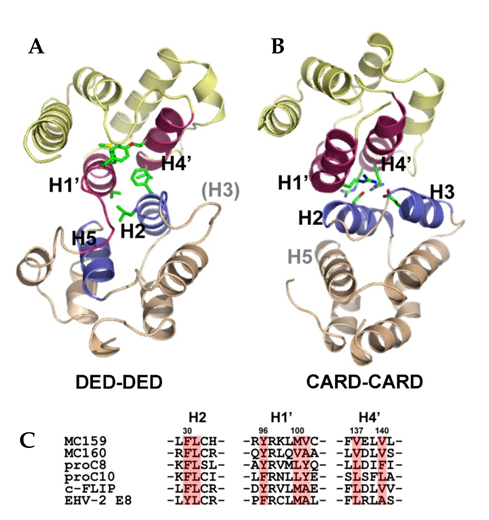
- Suppression of apoptosis is one of the hallmarks of carcinogenesis. Tumor cells endure apoptotic pressure by overexpressing several antiapoptotic proteins, and FLICE inhibitory protein (FLIP) is one of the important antiapoptotic proteins that have been shown to be overexpressed in various primary tumor cells. FLIP has two death-effector domains in tandem, mimicking the prodomain of procaspase-8. It is recruited to Fadd in death-inducing signaling complex, thereby preventing the activation of procaspase-8. To date, three isoforms of human cytosolic FLIP (c-FLIP) and six viral homologs (v-FLIP) have been identified. Recently, the crystal structure of v-FLIP MC159 was determined for the first time as an atomic-detail FLIP structure, which revealed that two death effector domains are packed tightly against each other mainly through conserved hydrophobic interactions. The overexpression of c-FLIP in tumor cells has been shown to be the determinant of the tumor's resistance to death ligands such as FasL and TRAIL. It has also been shown that the down-regulation of c-FLIP results in sensitizing resistant tumor cells. Therefore, the agents directly targeting c-FLIP at mRNA and protein levels are expected to be developed in near future and tested for the potential as a new class of anti-cancer drugs.
MeSH Terms
Figure
Reference
-
1. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972. 26:239–257.
Article2. Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001. 1:50–58.
Article3. Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980. 68:251–306.
Article4. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000. 100:57–70.
Article5. Wajant H. Targeting the FLICE Inhibitory Protein (FLIP) in cancer therapy. Mol Interv. 2003. 3:124–127.
Article6. Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005. 5:876–885.
Article7. Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004. 23:2950–2966.
Article8. Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005. 106:408–418.
Article9. Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005. 25:31–58.
Article10. Hengartner MO. The biochemistry of apoptosis. Nature. 2000. 407:770–776.
Article11. Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004. 117:855–858.12. Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996. 85:817–827.
Article13. Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, et al. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996. 271:4961–4965.
Article14. Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995. 14:5579–5588.
Article15. Fischer U, Jänicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003. 10:76–100.
Article16. Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006. 12:440–450.
Article17. French LE, Tschopp J. Protein-based therapeutic approaches targeting death receptors. Cell Death Differ. 2003. 10:117–123.
Article18. Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995. 3:673–682.
Article19. MacFarlane M. TRAIL-induced signalling and apoptosis. Toxicol Lett. 2003. 139:89–97.
Article20. Lahm A, Paradisi A, Green DR, Melino G. Death fold domain interaction in apoptosis. Cell Death Differ. 2003. 10:10–12.
Article21. Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997. 386:517–521.
Article22. Bertin J, Armstrong RC, Ottilie S, Martin DA, Wang Y, Banks S, et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci U S A. 1997. 94:1172–1176.
Article23. Hu S, Vincenz C, Buller M, Dixit VM. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem. 1997. 272:9621–9624.
Article24. Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997. 388:190–195.
Article25. Hu S, Vincenz C, Ni J, Gentz R, Dixit VM. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J Biol Chem. 1997. 272:17255–17257.
Article26. Djerbi M, Darreh-Shori T, Zhivotovsky B, Grandien A. Characterization of the human FLICE-inhibitory protein locus and comparison of the anti-apoptotic activity of four different flip isoforms. Scand J Immunol. 2001. 54:180–189.
Article27. Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005. 280:14507–14513.28. Yang JK, Wang L, Zheng L, Wan F, Ahmed M, Lenardo MJ, et al. Crystal structure of MC159 reveals molecular mechanism of DISC assembly and FLIP inhibition. Mol Cell. 2005. 20:939–949.
Article29. Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000. 12:633–642.
Article30. Goltsev YV, Kovalenko AV, Arnold E, Varfolomeev EE, Brodianskii VM, Wallach D. CASH, a novel caspase homologue with death effector domains. J Biol Chem. 1997. 272:19641–19644.
Article31. Han DK, Chaudhary PM, Wright ME, Friedman C, Trask BJ, Riedel RT, et al. MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc Natl Acad Sci U S A. 1997. 94:11333–11338.
Article32. Inohara N, Koseki T, Hu Y, Chen S, Núñez G. CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis. Proc Natl Acad Sci U S A. 1997. 94:10717–10722.
Article33. Shu HB, Halpin DR, Goeddel DV. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity. 1997. 6:751–763.
Article34. Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002. 21:3704–3714.
Article35. Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000. 10:640–648.
Article36. Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000. 275:10838–10844.37. Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol. 2004. 24:2627–2636.
Article38. Golks A, Brenner D, Krammer PH, Lavrik IN. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med. 2006. 203:1295–1305.39. Chaudhary PM, Eby MT, Jasmin A, Kumar A, Liu L, Hood L. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene. 2000. 19:4451–4460.
Article40. Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002. 419:395–399.
Article41. Su H, Bidère N, Zheng L, Cubre A, Sakai K, Dale J, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005. 307:1465–1468.
Article42. Peter ME. The flip side of FLIP. Biochem J. 2004. 382(Pt 2):e1–e3.
Article43. Park SJ, Kim YY, Ju JW, Han BG, Park SI, Park BJ. Alternative splicing variants of c-FLIP transduce the differential signal through the Raf or TRAF2 in TNF-induced cell proliferation. Biochem Biophys Res Commun. 2001. 289:1205–1210.
Article44. Li FY, Jeffrey PD, Yu JW, Shi Y. Crystal structure of a viral FLIP: insights into FLIP-mediated inhibition of death receptor signaling. J Biol Chem. 2006. 281:2960–2968.45. Eberstadt M, Huang B, Chen Z, Meadows RP, Ng SC, Zheng L, et al. NMR structure and mutagenesis of the FADD (Mort1) death-effector domain. Nature. 1998. 392:941–945.
Article46. Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature. 1996. 384:638–641.
Article47. Qin H, Srinivasula SM, Wu G, Fernandes-Alnemri T, Alnemri ES, Shi Y. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature. 1999. 399:549–557.
Article48. Tibbetts MD, Zheng L, Lenardo MJ. The death effector domain protein family: regulators of cellular homeostasis. Nat Immunol. 2003. 4:404–409.
Article49. Muppidi JR, Lobito AA, Ramaswamy M, Yang JK, Wang L, Wu H, et al. Homotypic FADD interactions through a conserved RXDLL motif are required for death receptor-induced apoptosis. Cell Death Differ. 2006. 13:1641–1650.
Article50. Garvey TL, Bertin J, Siegel RM, Wang GH, Lenardo MJ, Cohen JI. Binding of FADD and caspase-8 to molluscum contagiosum virus MC159 v-FLIP is not sufficient for its antiapoptotic function. J Virol. 2002. 76:697–706.
Article51. Carrington PE, Sandu C, Wei Y, Hill JM, Morisawa G, Huang T, et al. The structure of FADD and its mode of interaction with procaspase-8. Mol Cell. 2006. 22:599–610.
Article52. Hernandez A, Wang QD, Schwartz SA, Evers BM. Sensitization of human colon cancer cells to TRAIL-mediated apoptosis. J Gastrointest Surg. 2001. 5:56–65.
Article53. Ryu BK, Lee MG, Chi SG, Kim YW, Park JH. Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol. 2001. 194:15–19.
Article54. Korkolopoulou P, Saetta AA, Levidou G, Gigelou F, Lazaris A, Thymara I, et al. c-FLIP expression in colorectal carcinomas: association with Fas/FasL expression and prognostic implications. Histopathology. 2007. 51:150–156.
Article55. Ullenhag GJ, Mukherjee A, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res. 2007. 13:5070–5075.
Article56. Lee SH, Kim HS, Kim SY, Lee YS, Park WS, Kim SH, et al. Increased expression of FLIP, an inhibitor of Fas-mediated apoptosis, in stomach cancer. APMIS. 2003. 111:309–314.
Article57. Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, et al. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003. 94:1066–1073.
Article58. Zhou XD, Yu JP, Liu J, Luo HS, Chen HX, Yu HG. Overexpression of cellular FLICE-inhibitory protein (FLIP) in gastric adenocarcinoma. Clin Sci (Lond). 2004. 106:397–405.
Article59. Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Fujimura T, et al. Human pancreatic cancer cells disable function of Fas receptors at several levels in Fas signal transduction pathway. Int J Oncol. 2001. 18:311–316.
Article60. Mori T, Doi R, Toyoda E, Koizumi M, Ito D, Kami K, et al. Regulation of the resistance to TRAIL-induced apoptosis as a new strategy for pancreatic cancer. Surgery. 2005. 138:71–77.
Article61. Thomas RK, Kallenborn A, Wickenhauser C, Schultze JL, Draube A, Vockerodt M, et al. Constitutive expression of c-FLIP in Hodgkin and Reed-Sternberg cells. Am J Pathol. 2002. 160:1521–1528.
Article62. Dutton A, O'Neil JD, Milner AE, Reynolds GM, Starczynski J, Crocker J, et al. Expression of the cellular FLICE-inhibitory protein (c-FLIP) protects Hodgkin's lymphoma cells from autonomous Fas-mediated death. Proc Natl Acad Sci U S A. 2004. 101:6611–6616.
Article63. Mathas S, Lietz A, Anagnostopoulos I, Hummel F, Wiesner B, Janz M, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med. 2004. 199:1041–1052.
Article64. Olsson A, Diaz T, Aguilar-Santelises M, Osterborg A, Celsing F, Jondal M, et al. Sensitization to TRAIL-induced apoptosis and modulation of FLICE-inhibitory protein in B chronic lymphocytic leukemia by actinomycin D. Leukemia. 2001. 15:1868–1877.
Article65. MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, et al. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene. 2002. 21:6809–6818.
Article66. Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998. 161:2833–2840.67. Bullani RR, Huard B, Viard-Leveugle I, Byers HR, Irmler M, Saurat JH, et al. Selective expression of FLIP in malignant melanocytic skin lesions. J Invest Dermatol. 2001. 117:360–364.
Article68. Xiao CW, Yan X, Li Y, Reddy SA, Tsang BK. Resistance of human ovarian cancer cells to tumor necrosis factor alpha is a consequence of nuclear factor kappaB-mediated induction of Fas-associated death domain-like interleukin-1beta-converting enzyme-like inhibitory protein. Endocrinology. 2003. 144:623–630.
Article69. Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004. 23:6997–7004.
Article70. Mezzanzanica D, Balladore E, Turatti F, Luison E, Alberti P, Bagnoli M, et al. CD95-mediated apoptosis is impaired at receptor level by cellular FLICE-inhibitory protein (long form) in wild-type p53 human ovarian carcinoma. Clin Cancer Res. 2004. 10:5202–5214.
Article71. Wang W, Wang S, Song X, Sima N, Xu X, Luo A, et al. The relationship between c-FLIP expression and human papillomavirus E2 gene disruption in cervical carcinogenesis. Gynecol Oncol. 2007. 105:571–577.
Article72. Korkolopoulou P, Goudopoulou A, Voutsinas G, Thomas-Tsagli E, Kapralos P, Patsouris E, et al. c-FLIP expression in bladder urothelial carcinomas: its role in resistance to Fas-mediated apoptosis and clinicopathologic correlations. Urology. 2004. 63:1198–1204.
Article73. Zhang X, Jin TG, Yang H, DeWolf WC, Khosravi-Far R, Olumi AF. Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res. 2004. 64:7086–7091.
Article74. Siegmund D, Hadwiger P, Pfizenmaier K, Vornlocher HP, Wajant H. Selective inhibition of FLICE-like inhibitory protein expression with small interfering RNA oligonucleotides is sufficient to sensitize tumor cells for TRAIL-induced apoptosis. Mol Med. 2002. 8:725–732.
Article75. Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, Dicker DT, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004. 24:8541–8555.
Article76. Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000. 6:335–346.77. Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999. 190:1025–1032.
Article78. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999. 104:155–162.
Article79. Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999. 5:157–163.
Article80. Arkin M. Protein-protein interactions and cancer: small molecules going in for the kill. Curr Opin Chem Biol. 2005. 9:317–324.
Article81. Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997. 275:983–986.
Article82. Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T, et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000. 408:1004–1008.
Article83. Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000. 408:1008–1012.
Article84. Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996. 274:948–953.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of c-FLIP in Gastric Cancer and its Relation to Tumor Cell Proliferation and Apoptosis
- Target Controlled Infusion
- Toll-like receptor 9-mediated inhibition of apoptosis occurs through suppression of FoxO3a activity and induction of FLIP expression
- Specificity of Intracellular Trans-Splicing Reaction by hTERT-Targeting Group I Intron
- Target Therapy for Colorectal Cancer