Yonsei Med J.
2009 Apr;50(2):182-188.
Effect of beta2-Adrenergic Receptor Polymorphism in Asthma Control of Patients Receiving Combination Treatment
- Affiliations
-
- 1Department of Allergy and Rheumatology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Respiratory Medicine and Allergy, Dankook University School of Medicine, Cheonan, Korea.
- 3Department of Mathematics, Ajou University, Suwon, Korea.
- 4Infection, Inflammation, and Repair Division, School of Medicine, University of Southampton, Southampton, UK.
Abstract
- PURPOSE
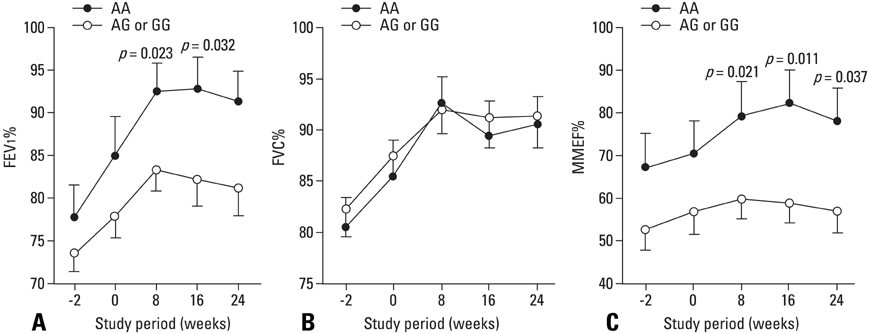
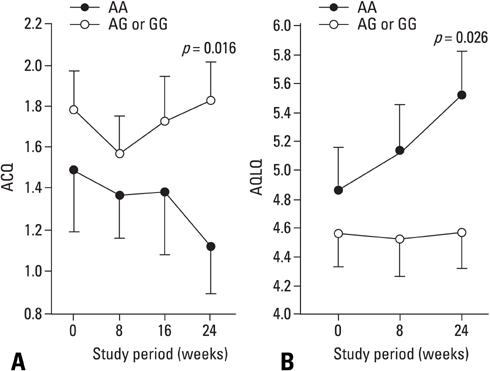
Combination treatment of inhaled corticosteroid (ICS) plus long-acting beta2-agonist (LABA) is widely used as a maintenance regimen for the management of asthma. This study evaluated the effect of the beta2-adrenergic receptor (ADRB2) polymorphism on lung function and asthma control with regular use of combination treatment of an inhaled ICS plus LABA. MATERIALS AND METHODS: 43 Korean asthmatics who were symptomatic despite regular ICS use for at least 3 months were enrolled. For a 2-week run-in period, they received ICS (budesonide 800 microgram/day) plus terbutaline (5 microgram prn). as needed. During the 24-week active treatment period, they received budesonide 160 microgram and formoterol 4.5 microgram b.i.d. as maintenance and rescue medication. Pulmonary function and quality of life scores were monitored every 8 weeks; morning/evening peak expiratory flow meter (PEFR) was recorded daily. Patients were genotyped for ADRB2 Arg16Gly using single base extension methodology. RESULTS: During the run-in period, there were no significant between-group differences in lung function; after 8 weeks of active treatment, Arg/Arg patients had significantly higher forced expiratory volume in 1 secord (FEV1) and maximal mid-expiratory flow (MMEF) (p = 0.023 and p = 0.021, respectively), and better asthma control and quality of life after 24 weeks (p = 0.016 and p = 0.028, respectively). During treatment, there was a greater improvement in morning/evening PEFR in Arg/Arg patients. CONCLUSION: Asthmatic patients with the Arg/Arg genotype at codon 16 of ADRB2 achieve better asthma control with long-term regular use of combined budesonide and formoterol treatment, suggesting that the ADRB2 genotype may dictate choice of treatment strategy.
MeSH Terms
Figure
Reference
-
1. O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005. 171:129–136.2. Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997. 337:1405–1411.3. National Instritutes of Health, National Heart, Lung and Blood Institute. Global initiative for asthma (gina) global strategy for asthma managemetn and prevention. 2006.4. Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000. 162:75–80.
Article5. Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004. 364:1505–1512.
Article6. Wechsler ME, Lehman E, Lazarus SC, Lemanske RF Jr, Boushey HA, Deykin A, et al. beta-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med. 2006. 173:519–526.
Article7. Palmer CN, Lipworth BJ, Lee S, Ismail T, Macgregor DF, Mukhopadhyay S. Arginine-16 beta2 adrenoceptor genotype predisposes to exacerbations in young asthmatics taking regular salmeterol. Thorax. 2006. 61:940–944.
Article8. Lee DK, Currie GP, Hall IP, Lima JJ, Lipworth BJ. The arginine-16 beta2-adrenoceptor polymorphism predisposes to bronchoprotective subsensitivity in patients treated with formoterol and salmeterol. Br J Clin Pharmacol. 2004. 57:68–75.
Article9. Cho SH, Oh SY, Bahn JW, Choi JY, Chang YS, Kim YK, et al. Association between bronchodilating response to short-acting beta-agonist and non-synonymous single-nucleotide polymorphisms of beta-adrenoceptor gene. Clin Exp Allergy. 2005. 35:1162–1167.
Article10. Taylor DR, Epton MJ, Kennedy MA, Smith AD, Iles S, Miller AL, et al. Bronchodilator response in relation to beta2-adrenoceptor haplotype in patients with asthma. Am J Respir Crit Care Med. 2005. 172:700–703.
Article11. Hancox RJ, Sears MR, Taylor DR. Polymorphism of the beta2-adrenoceptor and the response to long-term beta2-agonist therapy in asthma. Eur Respir J. 1998. 11:589–593.12. Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax. 2000. 55:762–767.
Article13. Bleecker ER, Yancey SW, Baitinger LA, Edwards LD, Klotsman M, Anderson WH, et al. Salmeterol response is not affected by beta2-adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol. 2006. 118:809–816.
Article14. Bleecker ER, Postma DS, Lawrance RM, Meyers DA, Ambrose HJ, Goldman M. Effect of ADRB2 polymorphisms on response to longacting beta2-agonist therapy: a pharmacogenetic analysis of two randomised studies. Lancet. 2007. 370:2118–2125.
Article15. Choudhry S, Ung N, Avila PC, Ziv E, Nazario S, Casal J, et al. Pharmacogenetic differences in response to albuterol between Puerto Ricans and Mexicans with asthma. Am J Respir Crit Care Med. 2005. 171:563–570.
Article16. Rho HJ, Park MS, Park CW, Yun YY, Park JW, Hong CS, et al. Factors influencing quality of life of asthmatic patients in Korea. J Asthma Allergy Clin Immunol. 2000. 20:209–221.17. Liggett SB. Polymorphisms of the beta2-adrenergic receptor and asthma. Am J Respir Crit Care Med. 1997. 156:S156–S162.18. Holloway JW, Dunbar PR, Riley GA, Sawyer GM, Fitzharris PF, Pearce N, et al. Association of beta2-adrenergic receptor polymorphisms with severe asthma. Clin Exp Allergy. 2000. 30:1097–1103.19. Contopoulos-Ioannidis DG, Manoli EN, Ioannidis JP. Meta-analysis of the association of beta2-adrenergic receptor polymorphisms with asthma phenotypes. J Allergy Clin Immunol. 2005. 115:963–972.20. Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993. 8:334–339.
Article21. Martinez FD, Graves PE, Baldini M, Solomon S, Erickson R. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J Clin Invest. 1997. 100:3184–3188.
Article22. Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, et al. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000. 97:10483–10488.
Article23. Munakata M, Harada Y, Ishida T, Saito J, Nagabukuro A, Matsushita H, et al. Molecular-based haplotype analysis of the beta 2-adrenergic receptor gene (ADRB2) in Japanese asthmatic and non-asthmatic subjects. Allergol Int. 2006. 55:191–198.
Article24. Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994. 33:9414–9419.
Article25. Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995. 13:25–33.
Article26. Green SA, Rathz DA, Schuster AJ, Liggett SB. The Ile164 beta(2)-adrenoceptor polymorphism alters salmeterol exosite binding and conventional agonist coupling to G(s). Eur J Pharmacol. 2001. 421:141–147.
Article27. Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of the human beta 2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem. 1993. 268:23116–23121.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- beta2 adrenergic receptor polymrphism
- Association between beta2 adrenoceptor polymorphisms and atopy/serum IgE in asthmatic patients
- Association of beta2-adrenoceptor Polymorphisms with Baseline FEV1 in Asthmatics
- The Relationship Between Leptin and Adrenergic Receptors Genes Polymorphisms and Changes in Production of Osteoprotegerin and Soluble Receptor Activator of NF-kappaB by Whole Blood Cells after Hormone Therapy
- Pharmacogenomic Approaches to Asthma Treatment