Allergy Asthma Immunol Res.
2010 Jul;2(3):177-182. 10.4168/aair.2010.2.3.177.
Pharmacogenomic Approaches to Asthma Treatment
- Affiliations
-
- 1Division of Asthma, Allergy and Clinical Immunology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. shcho@plaza.snu.ac.kr
- KMID: 2133805
- DOI: http://doi.org/10.4168/aair.2010.2.3.177
Abstract
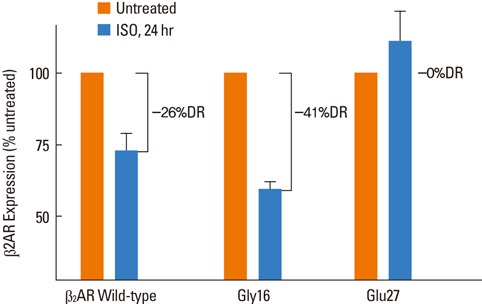
- Major classes of medication in asthma management include bronchodilating beta2-agonists, anti-inflammatory inhaled corticosteroids, leukotriene modifiers and theophyllines. However, all asthmatics do not respond to the same extent to a given medication. Available data suggest that a substantial range of individual variability, as much as 70%, may be due to genetic characteristics of each patient. Pharmacogenomics offers the potential to optimize medications for individual asthmatics by using genetic information to improve efficacy or avoid adverse effects. The best-studied case of the potential contribution of pharmacogenomics to treatment response in asthma comes from studies on human beta2 adrenergic receptors. In addition, genetic variation in beta2-adrenergic receptor (Arg16Gly) may predict response to anticholinergics for the treatment of asthma. In case of inhaled corticosteroids, a recent investigation using a traditional SNP-based approach identified a gene for corticotropin releasing hormone receptor 1 as a potential marker of response. Another major pathway that has been investigated is the pathway underlying response to cysteinyl leukotriene receptor antagonist. It is likely that in the near future, pharmacogenomic approaches based on individual genetic information will be introduced into an asthma treatment guideline and this guideline will allow us to identify those who have the best chance to respond to a specific medication.
Keyword
MeSH Terms
-
Adrenal Cortex Hormones
Asthma
Cholinergic Antagonists
Genes, vif
Genetic Variation
Humans
Pharmacogenetics
Receptors, Adrenergic
Receptors, Corticotropin-Releasing Hormone
Receptors, Leukotriene
Adrenal Cortex Hormones
Cholinergic Antagonists
Receptors, Adrenergic
Receptors, Corticotropin-Releasing Hormone
Receptors, Leukotriene
Figure
Reference
-
1. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008. 31:143–178.2. Lugogo NL, Kraft M. Epidemiology of asthma. Clin Chest Med. 2006. 27:1–15. v.3. Jee HM, Kim KW, Kim CS, Sohn MH, Shin DC, Kim KE. Prevalence of asthma, rhinitis and eczema in korean children using the International Study Of Asthma and Allergies In Childhood (ISAAC) questionnaires. Pediatr Allergy Respir Dis. 2009. 19:165–172. Korean.4. Lee SI. Prevalence of childhood asthma in Korea: international study of asthma and allergies in childhood. Allergy Asthma Immunol Res. 2010. 2:61–64.5. Cho SH, Park HW, Rosenberg DM. The current status of asthma in Korea. J Korean Med Sci. 2006. 21:181–187.6. Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE. GOAL Investigators Group. Can guideline-defined asthma control be achieved The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004. 170:836–844.7. Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000. 56:1054–1070.8. Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol. 2006. 117:18–24. quiz 5.9. Maxwell TJ, Ameyaw MM, Pritchard S, Thornton N, Folayan G, Githang'a J, Indalo A, Tariq M, Mobarek A, Evans DA, Ofori-Adjei D, Templeton AR, McLeod HL. Beta-2 adrenergic receptor genotypes and haplotypes in different ethnic groups. Int J Mol Med. 2005. 16:573–580.10. Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994. 33:9414–9419.11. Cho SH, Oh SY, Bahn JW, Choi JY, Chang YS, Kim YK, Min KU, Kim YY. Association between bronchodilating response to short-acting beta-agonist and non-synonymous single-nucleotide polymorphisms of beta-adrenoceptor gene. Clin Exp Allergy. 2005. 35:1162–1167.12. Lima JJ, Thomason DB, Mohamed MH, Eberle LV, Self TH, Johnson JA. Impact of genetic polymorphisms of the beta2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther. 1999. 65:519–525.13. Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, Kraft M, Kunselman S, Lazarus SC, Lemanske RF, Martin RJ, McLean DE, Peters SP, Silverman EK, Sorkness CA, Szefler SJ, Weiss ST, Yandava CN. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000. 162:75–80.14. Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax. 2000. 55:762–767.15. Wechsler ME, Lehman E, Lazarus SC, Lemanske RF Jr, Boushey HA, Deykin A, Fahy JV, Sorkness CA, Chinchilli VM, Craig TJ, Di-Mango E, Kraft M, Leone F, Martin RJ, Peters SP, Szefler SJ, Liu W, Israel E. beta-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med. 2006. 173:519–526.16. Bleecker ER, Postma DS, Lawrance RM, Meyers DA, Ambrose HJ, Goldman M. Effect of ADRB2 polymorphisms on response to longacting beta2-agonist therapy: a pharmacogenetic analysis of two randomised studies. Lancet. 2007. 370:2118–2125.17. Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2006. 117:522–543.18. Bray PJ, Cotton RG. Variations of the human glucocorticoid receptor gene (NR3C1): pathological and in vitro mutations and polymorphisms. Hum Mutat. 2003. 21:557–568.19. Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, Liggett SB, Gelfand EW, Rosenwasser LJ, Richter B, Israel E, Wechsler M, Gabriel S, Altshuler D, Lander E, Drazen J, Weiss ST. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004. 13:1353–1359.20. Ito K, Caramori G, Lim S, Oates T, Chung KF, Barnes PJ, Adcock IM. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med. 2002. 166:392–396.21. Bhavsar P, Ahmad T, Adcock IM. The role of histone deacetylases in asthma and allergic diseases. J Allergy Clin Immunol. 2008. 121:580–584.22. Fischer DD, Cai R, Bhatia U, Asselbergs FA, Song C, Terry R, Trogani N, Widmer R, Atadja P, Cohen D. Isolation and characterization of a novel class II histone deacetylase, HDAC10. J Biol Chem. 2002. 277:6656–6666.23. Drazen JM, Yandava CN, Dube L, Szczerback N, Hippensteel R, Pillari A, Israel E, Schork N, Silverman ES, Katz DA, Drajesk J. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999. 22:168–170.24. Lima JJ, Zhang S, Grant A, Shao L, Tantisira KG, Allayee H, Wang J, Sylvester J, Holbrook J, Wise R, Weiss ST, Barnes K. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006. 173:379–385.25. Asano K, Shiomi T, Hasegawa N, Nakamura H, Kudo H, Matsuzaki T, Hakuno H, Fukunaga K, Suzuki Y, Kanazawa M, Yamaguchi K. Leukotriene C4 synthase gene A(-444)C polymorphism and clinical response to a CYS-LT(1) antagonist, pranlukast, in Japanese patients with moderate asthma. Pharmacogenetics. 2002. 12:565–570.26. Kim SH, Ye YM, Hur GY, Lee SK, Sampson AP, Lee HY, Park HS. Cys-LTR1 promoter polymorphism and requirement for leukotriene receptor antagonist in aspirin-intolerant asthma patients. Pharmacogenomics. 2007. 8:1143–1150.27. Klotsman M, York TP, Pillai SG, Vargas-Irwin C, Sharma SS, van den Oord EJ, Anderson WH. Pharmacogenetics of the 5-lipoxygenase biosynthetic pathway and variable clinical response to montelukast. Pharmacogenet Genomics. 2007. 17:189–196.28. Currie GP, Lima JJ, Sylvester JE, Lee DK, Cockburn WJ, Lipworth BJ. Leukotriene C4 synthase polymorphisms and responsiveness to leukotriene antagonists in asthma. Br J Clin Pharmacol. 2003. 56:422–426.29. Lee SY, Kim HB, Kim JH, Kim BS, Kang MJ, Jang SO, Seo HJ, Hong SJ. Responsiveness to montelukast is associated with bronchial hyperresponsiveness and total immunoglobulin E but not polymorphisms in the leukotriene C4 synthase and cysteinyl leukotriene receptor 1 genes in Korean children with exercise-induced asthma (EIA). Clin Exp Allergy. 2007. 37:1487–1493.30. Kim JH, Lee SY, Kim HB, Jin HS, Yu JH, Kim BJ, Kim BS, Kang MJ, Jang SO, Hong SJ. TBXA2R gene polymorphism and responsiveness to leukotriene receptor antagonist in children with asthma. Clin Exp Allergy. 2008. 38:51–59.31. Kang MJ, Lee SY, Kim HB, Yu J, Kim BJ, Choi WA, Jang SO, Hong SJ. Association of IL-13 polymorphisms with leukotriene receptor antagonist drug responsiveness in Korean children with exercise-induced bronchoconstriction. Pharmacogenet Genomics. 2008. 18:551–558.32. Iwamoto H, Yokoyama A, Shiota N, Shoda H, Haruta Y, Hattori N, Kohno N. Tiotropium bromide is effective for severe asthma with noneosinophilic phenotype. Eur Respir J. 2008. 31:1379–1380.33. Kapoor AS, Olsen SR, O'Hara C, Puttagunta L, Vethanayagam D. The efficacy of tiotropium as a steroid-sparing agent in severe asthma. Can Respir J. 2009. 16:99–101.34. Park HW, Yang MS, Park CS, Kim TB, Moon HB, Min KU, Kim YY, Cho SH. Additive role of tiotropium in severe asthmatics and Arg-16Gly in ADRB2 as a potential marker to predict response. Allergy. 2009. 64:778–783.