Clin Exp Otorhinolaryngol.
2017 Sep;10(3):248-253. 10.21053/ceo.2016.01081.
Efficacy and Safety of Guardcel Nasal Packing After Endoscopic Sinus Surgery: A Prospective, Single-Blind, Randomized Controlled Study
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea. lhman@korea.ac.kr
- 2Division of Brain Korea 21 Program for Biomedical Science, Korea University College of Medicine, Seoul, Korea.
- 3In vitro Diagnostic Medical Devices Support Center, Korea University Guro Hospital, Seoul, Korea.
- KMID: 2395739
- DOI: http://doi.org/10.21053/ceo.2016.01081
Abstract
OBJECTIVES
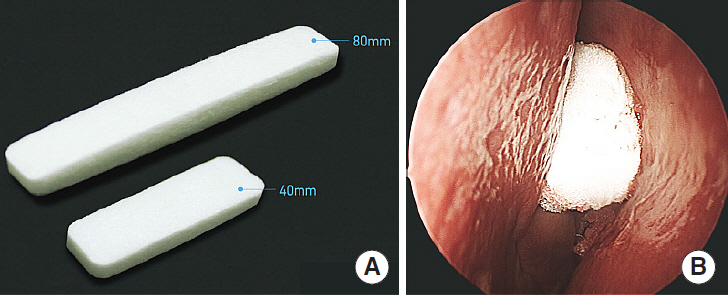
Nasal packing after endoscopic sinus surgery is frequently used to control postoperative bleeding, enhance the wound healing process, and prevent lateralization of the middle turbinate, which causes insufficient ventilation. Many biodegradable materials have been developed to reduce pain and mucosal damage during packing removal. The purpose of this study was to compare the efficacy of Guardcel (Genewel Co.) middle meatal packing with a traditional nonabsorbable middle meatal packing, Merocel (Medtronic Xomed), on wound healing and patient satisfaction.
METHODS
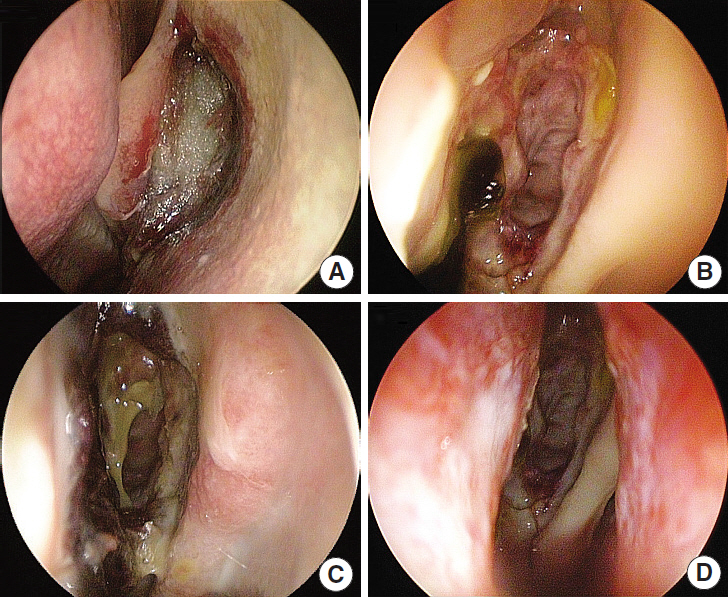
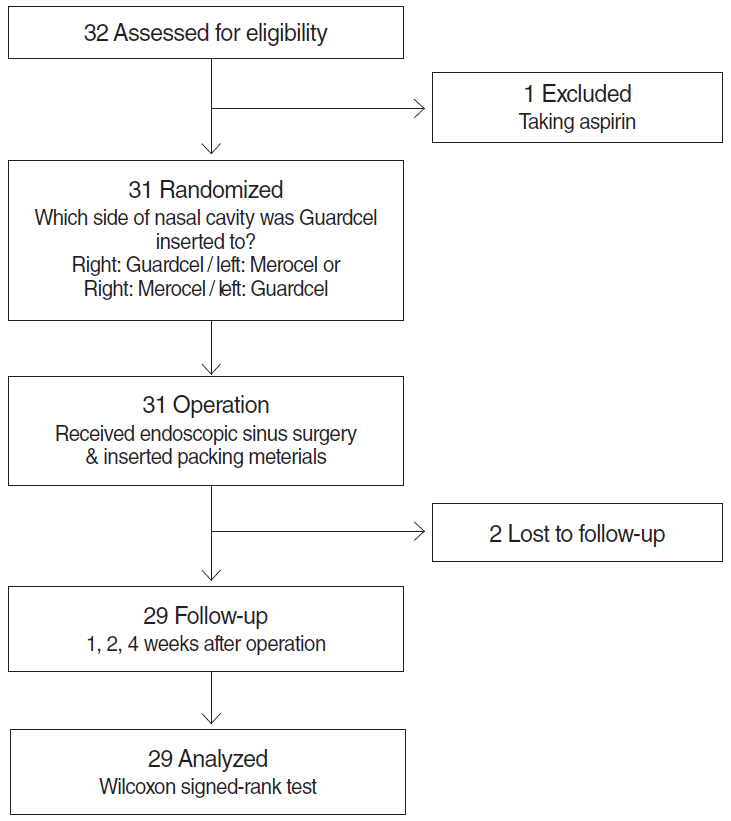
In this prospective, single-blind, randomized controlled study, we enrolled 32 consecutive patients (64 nostrils) undergoing bilateral endoscopic sinus surgery at Korea University Guro Hospital from February 2015 to August 2015. Guardcel and Merocel were inserted postoperatively into a randomly assigned side. Objective findings about bleeding, hemostasis, adhesion, and infection were evaluated with nasal endoscopy. Patients' symptoms including pain and nasal obstruction were evaluated with a visual analog scale. Each evaluation was done at 2-3 days, 1 week, 2 weeks, and 4 weeks after surgery.
RESULTS
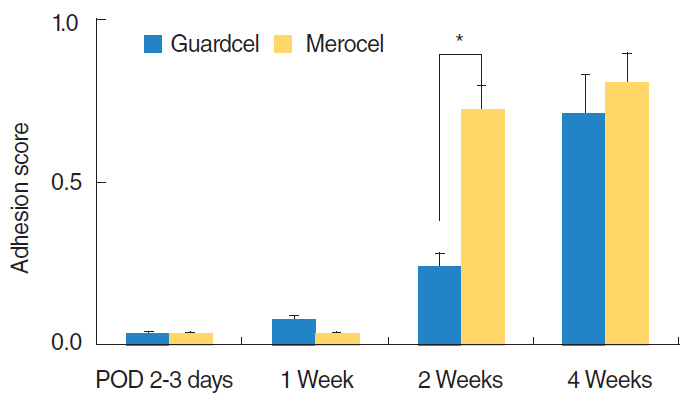
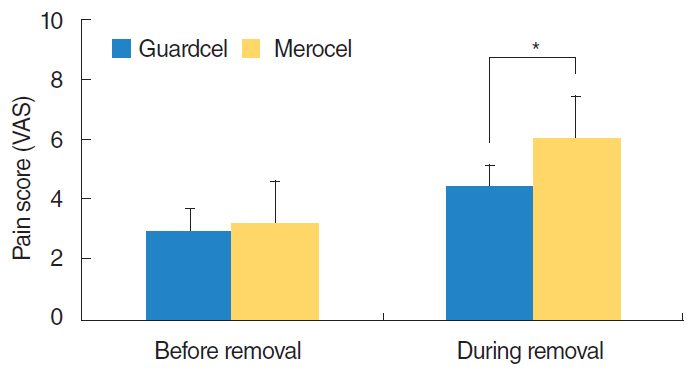
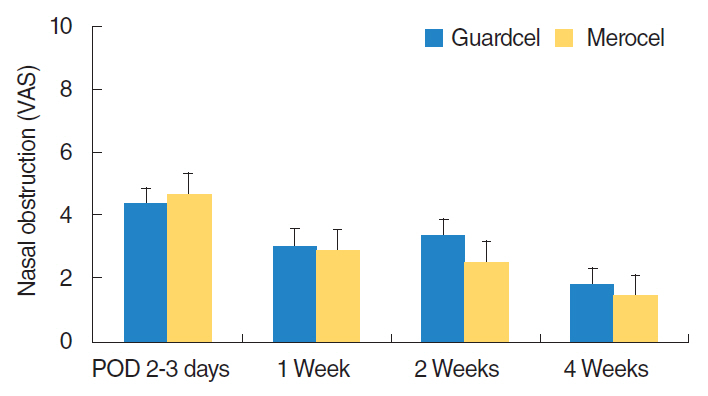
At 2-3 days after endoscopic sinus surgery, the Guardcel side had a significantly less hemostasis time than the Merocel side (P=0.001). During this period, the pain during packing removal was significantly lower on the Guardcel-inserted side than the Merocel-inserted side (P=0.002). At two weeks after surgery, the adhesion score on the Guardcel side was significantly lower than that of the Merocel side (P=0.011). Other parameters during the study follow-up periods were not statistically significant. There were no severe adverse reactions.
CONCLUSION
Guardcel, a newly developed packing material, appeared to shorten the hemostasis time and reduce pain sensation at 2-3 days after surgery; it also prevented adhesion formation 2 weeks after surgery when compared with the control. Guardcel can be an effective and safe candidate to replace conventional packing materials after endoscopic sinus surgery.
MeSH Terms
Figure
Reference
-
1. Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003; Sep. 129(3 Suppl):S1–32.
Article2. Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011; May-Jun. 25(3):117–21.
Article3. Govindaraj S, Adappa ND, Kennedy DW. Endoscopic sinus surgery: evolution and technical innovations. J Laryngol Otol. 2010; Mar. 124(3):242–50.
Article4. Valentine R, Wormald PJ, Sindwani R. Advances in absorbable biomaterials and nasal packing. Otolaryngol Clin North Am. 2009; Oct. 42(5):813–28.
Article5. Yan M, Zheng D, Li Y, Zheng Q, Chen J, Yang B. Biodegradable nasal packings for endoscopic sinonasal surgery: a systematic review and meta-analysis. PLoS One. 2014; 9(12):e115458.
Article6. Berlucchi M, Castelnuovo P, Vincenzi A, Morra B, Pasquini E. Endoscopic outcomes of resorbable nasal packing after functional endoscopic sinus surgery: a multicenter prospective randomized controlled study. Eur Arch Otorhinolaryngol. 2009; Jun. 266(6):839–45.
Article7. Shoman N, Gheriani H, Flamer D, Javer A. Prospective, double-blind, randomized trial evaluating patient satisfaction, bleeding, and wound healing using biodegradable synthetic polyurethane foam (NasoPore) as a middle meatal spacer in functional endoscopic sinus surgery. J Otolaryngol Head Neck Surg. 2009; Feb. 38(1):112–8.8. Mo JH, Han DH, Shin HW, Cha W, Chang MY, Jin HR. No packing versus packing after endoscopic sinus surgery: pursuit of patients’ comfort after surgery. Am J Rhinol. 2008; Sep-Oct. 22(5):525–8.
Article9. Leunig A, Betz CS, Siedek V, Kastl KG. CMC packing in functional endoscopic sinus surgery: does it affect patient comfort. Rhinology. 2009; Mar. 47(1):36–40.10. Kastl KG, Betz CS, Siedek V, Leunig A. Effect of carboxymethylcellulose nasal packing on wound healing after functional endoscopic sinus surgery. Am J Rhinol Allergy. 2009; Jan-Feb. 23(1):80–4.
Article11. Wormald PJ, Boustred RN, Le T, Hawke L, Sacks R. A prospective single-blind randomized controlled study of use of hyaluronic acid nasal packs in patients after endoscopic sinus surgery. Am J Rhinol. 2006; Jan-Feb. 20(1):7–10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Effects of Guardcel® Nasal Packing on Endonasal Dacryocystorhinostomy
- Packing Materials for Nasal and Paranasal Sinus
- A Case of Maxillary Sinus Spindle Cell Sarcoma: Alert to Polyurethane Associated Malignancy
- The Effect of Triamcinolone-Soaked Gelfoam in Patients with Polypoid Mucosal Change after Endoscopic Sinus Surgery
- The Usefulness of Latex-covered Merocel(R) Packing in Septal Surgery