Cancer Res Treat.
2017 Oct;49(4):1140-1152. 10.4143/crt.2016.491.
Engineering of Anti-CD133 Trispecific Molecule Capable of Inducing NK Expansion and Driving Antibody-Dependent Cell-Mediated Cytotoxicity
- Affiliations
-
- 1Department of Therapeutic Radiology-Radiation Oncology, University of Minnesota, Masonic Cancer Center, Minneapolis, MN, USA. valle001@umn.edu
- 2Department for Hematology and Oncology, Medicine Department 2, University Hospital of Tuebingen, University of Tuebingen, Tuebingen, Germany.
- 3Department of Medicine, Division of Hematology, Oncology, and Transplantation, Minneapolis, MN, USA.
- 4Department of Pharmacology, University of Minnesota, Minneapolis, MN, USA.
- KMID: 2394832
- DOI: http://doi.org/10.4143/crt.2016.491
Abstract
- PURPOSE
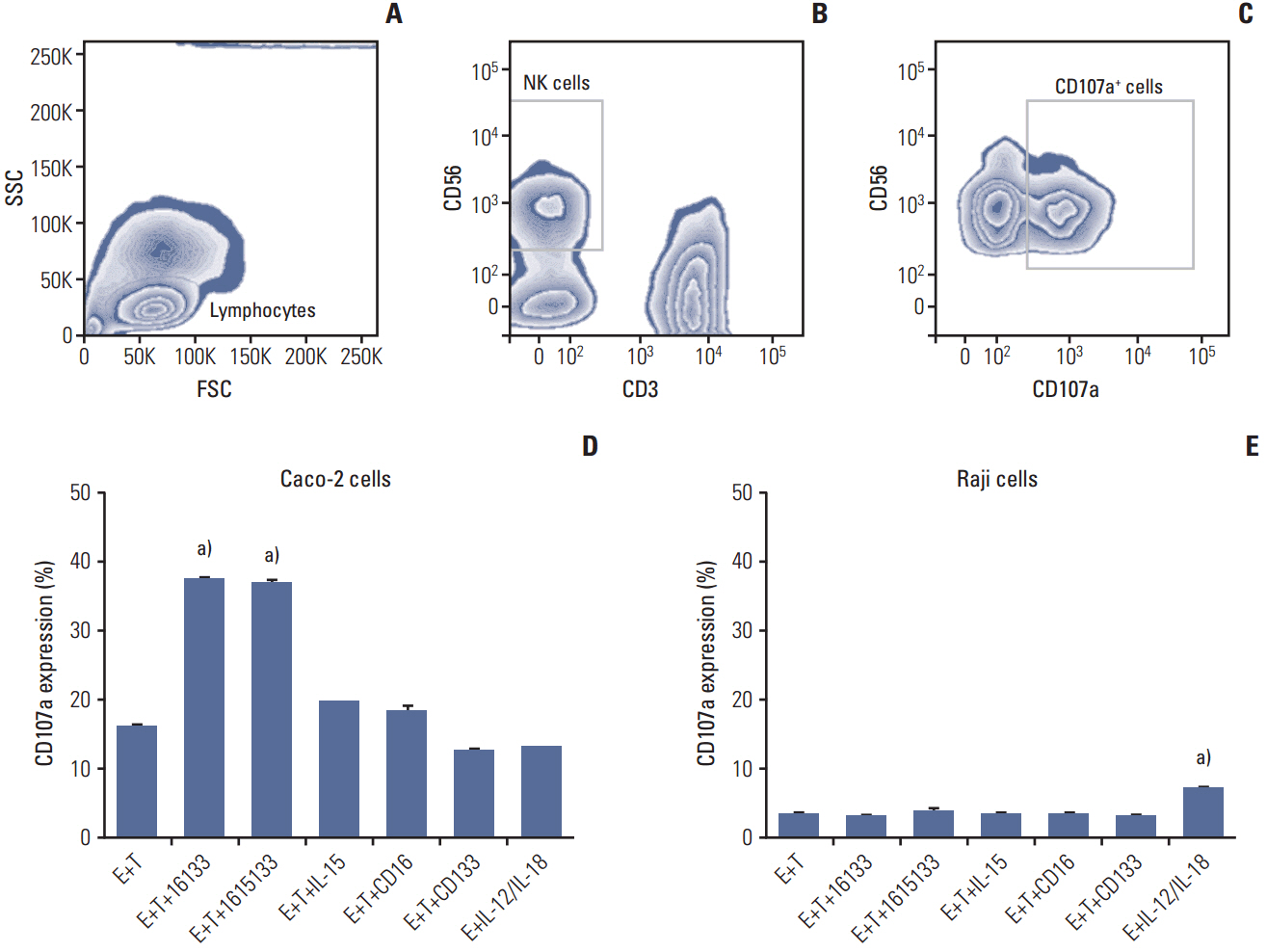
The selective elimination of cancer stem cells (CSCs) in tumor patients is a crucial goal because CSCs cause drug refractory relapse. To improve the current conventional bispecific immune-engager platform, a 16133 bispecific natural killer (NK) cell engager (BiKE), consisting of scFvs binding FcγRIII (CD16) on NK cells and CD133 on carcinoma cells, was first synthesized and a modified interleukin (IL)-15 crosslinker capable of stimulating NK effector cells was introduced.
MATERIALS AND METHODS
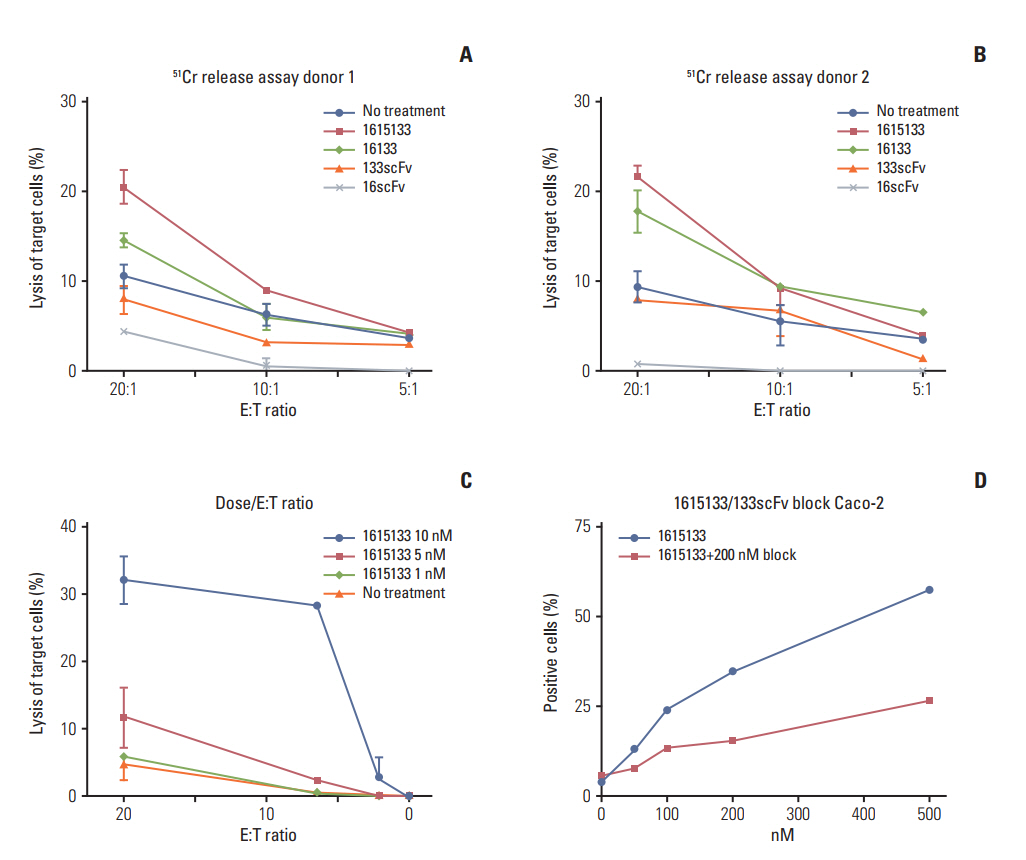
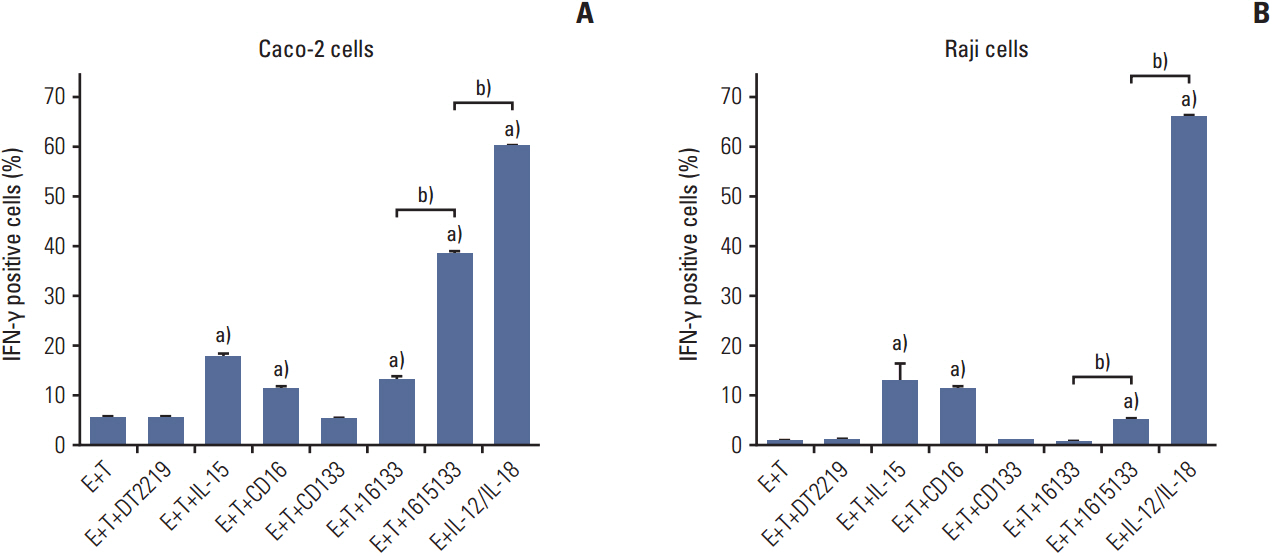
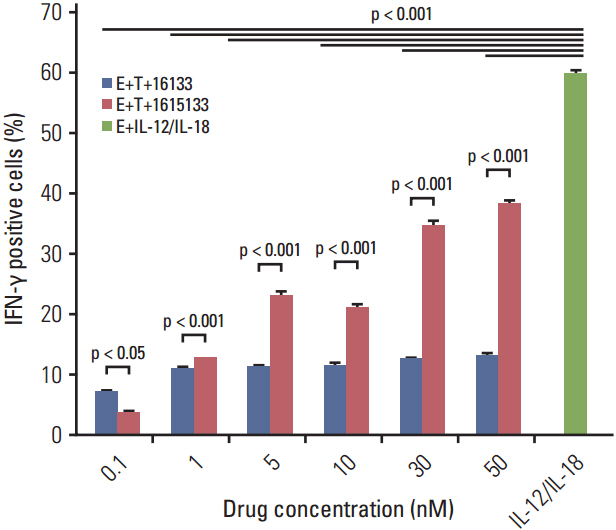
DNA shuffling and ligation techniques were used to assemble and synthesize the 1615133 trispecific NK cell engager (TriKE). The construct was tested for its specificity using flow cytometry, cytotoxic determinations using chromium release assays, and lytic degranulation. IL-15-mediated expansion was measured using flow-based proliferation assays. The level of interferon (IFN)-γ release was measured because of its importance in the anti-cancer response.
RESULTS
1615133 TriKE induced NK cell-mediated cytotoxicity and NK expansion far greater than that achieved with BiKE devoid of IL-15. The drug binding and induction of cytotoxic degranulation was CD133+ specific and the anti-cancer activity was improved by integrating the IL-15 cross linker. The NK cell-related cytokine release measured by IFN-γ detection was higher than that of BiKE. NK cytokine release studies showed that although the IFN-γ levels were elevated, they did not approach the levels achieved with IL-12/IL-18, indicating that release was not at the supraphysiologic level.
CONCLUSION
1615133 TriKE enhances the NK cell anti-cancer activity and provides a self-sustaining mechanism via IL-15 signaling. By improving the NK cell performance, the new TriKE represents a highly active drug against drug refractory relapse mediated by CSCs.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Kim Y, Joo KM, Jin J, Nam DH. Cancer stem cells and their mechanism of chemo-radiation resistance. Int J Stem Cells. 2009; 2:109–14.
Article2. Du Z, Qin R, Wei C, Wang M, Shi C, Tian R, et al. Pancreatic cancer cells resistant to chemoradiotherapy rich in “stem-celllike” tumor cells. Dig Dis Sci. 2011; 56:741–50.
Article3. Fabrizi E, di Martino S, Pelacchi F, Ricci-Vitiani L. Therapeutic implications of colon cancer stem cells. World J Gastroenterol. 2010; 16:3871–7.
Article4. Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008; 99:100–9.
Article5. Hashimoto K, Aoyagi K, Isobe T, Kouhuji K, Shirouzu K. Expression of CD133 in the cytoplasm is associated with cancer progression and poor prognosis in gastric cancer. Gastric Cancer. 2014; 17:97–106.
Article6. Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994; 180:1395–403.
Article7. Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999; 162:4511–20.8. Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001; 97:3146–51.
Article9. Childs RW, Berg M. Bringing natural killer cells to the clinic: ex vivo manipulation. Hematology Am Soc Hematol Educ Program. 2013; 2013:234–46.
Article10. Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002; 13:169–83.
Article11. Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010; 33:599–608.
Article12. Stein C, Kellner C, Kugler M, Reiff N, Mentz K, Schwenkert M, et al. Novel conjugates of single-chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. Br J Haematol. 2010; 148:879–89.
Article13. Swaminathan SK, Niu L, Waldron N, Kalscheuer S, Zellmer DM, Olin MR, et al. Identification and characterization of a novel scFv recognizing human and mouse CD133. Drug Deliv Transl Res. 2013; 3:143–51.
Article14. Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res. 2016; 22:3440–50.
Article15. Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2003; 63:803–43.16. Schmohl JU, Vallera DA. CD133, selectively targeting the root of cancer. Toxins (Basel). 2016; 8:E165.
Article17. McCall AM, Adams GP, Amoroso AR, Nielsen UB, Zhang L, Horak E, et al. Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/ neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. Mol Immunol. 1999; 36:433–45.18. Zhu X, Marcus WD, Xu W, Lee HI, Han K, Egan JO, et al. Novel human interleukin-15 agonists. J Immunol. 2009; 183:3598–607.
Article19. Schmohl JU, Gleason MK, Dougherty PR, Miller JS, Vallera DA. Heterodimeric bispecific single chain variable fragments (scFv) killer engagers (BiKEs) enhance NK-cell activity against CD133+ colorectal cancer cells. Target Oncol. 2016; 11:353–61.
Article20. Schmohl JU, Felices M, Taras E, Miller JS, Vallera DA. Enhanced ADCC and NK Cell activation of an anticarcinoma bispecific antibody by genetic insertion of a modified IL-15 cross-linker. Mol Ther. 2016; 24:1312–22.
Article21. Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977; 59:221–6.22. Roederer M. Interpretation of cellular proliferation data: avoid the panglossian. Cytometry A. 2011; 79:95–101.
Article23. Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012; 11:2674–84.
Article24. Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov. 2015; 14:487–98.
Article25. Connor JP, Felder M, Hank J, Harter J, Gan J, Gillies SD, et al. Ex vivo evaluation of anti-EpCAM immunocytokine huKS-IL2 in ovarian cancer. J Immunother. 2004; 27:211–9.
Article26. Munger W, DeJoy SQ, Jeyaseelan R Sr, Torley LW, Grabstein KH, Eisenmann J, et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol. 1995; 165:289–93.
Article27. Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015; 33:74–82.
Article28. Waldmann TA. Interleukin-15 in the treatment of cancer. Expert Rev Clin Immunol. 2014; 10:1689–701.
Article29. Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002; 13:95–109.30. Waldron NN, Barsky SH, Dougherty PR, Vallera DA. A bispecific EpCAM/CD133-targeted toxin is effective against carcinoma. Target Oncol. 2014; 9:239–49.
Article31. Waldron NN, Kaufman DS, Oh S, Inde Z, Hexum MK, Ohlfest JR, et al. Targeting tumor-initiating cancer cells with dCD133-KDEL shows impressive tumor reductions in a xenotransplant model of human head and neck cancer. Mol Cancer Ther. 2011; 10:1829–38.
Article32. Ohlfest JR, Zellmer DM, Panyam J, Swaminathan SK, Oh S, Waldron NN, et al. Immunotoxin targeting CD133(+) breast carcinoma cells. Drug Deliv Transl Res. 2013; 3:195–204.
Article33. Damek-Poprawa M, Volgina A, Korostoff J, Sollecito TP, Brose MS, O'Malley BW Jr, et al. Targeted inhibition of CD133+ cells in oral cancer cell lines. J Dent Res. 2011; 90:638–45.34. Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997; 90:5002–12.
Article35. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000; 95:952–8.
Article36. Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000; 97:14720–5.
Article37. Skubitz AP, Taras EP, Boylan KL, Waldron NN, Oh S, Panoskaltsis-Mortari A, et al. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol Oncol. 2013; 130:579–87.
Article38. Rutella S, Bonanno G, Marone M, De Ritis D, Mariotti A, Voso MT, et al. Identification of a novel subpopulation of human cord blood CD34-CD133-CD7-CD45+lineage- cells capable of lymphoid/NK cell differentiation after in vitro exposure to IL-15. J Immunol. 2003; 171:2977–88.39. Suuronen EJ, Wong S, Kapila V, Waghray G, Whitman SC, Mesana TG, et al. Generation of CD133+ cells from CD133-peripheral blood mononuclear cells and their properties. Cardiovasc Res. 2006; 70:126–35.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Natural Killer Cell Mediated Cytotoxicity and Antibody-Dependent Cell Mediated Cytotoxicity in Tuberculosis Patients (1)
- Optimization of Large-Scale Expansion and Cryopreservation of Human Natural Killer Cells for Anti-Tumor Therapy
- Correlation of Natural Killer(NK) Cell Activity,Antibody Dependent Cellular Cytotoxicity(ADCC), and Serum Zinc Level in Behçet's Disease
- Establishment of Antibody-dependant Cellular Cytotoxicity against Human Squamous Cell Carcinoma of Head and Neck and The Effect of Prostaglandin on Antibody-dependant Cellular Cytotoxicity
- Expansion and Activation of Natural Killer Cells for Cancer Immunotherapy