Cancer Res Treat.
2017 Oct;49(4):1127-1139. 10.4143/crt.2016.538.
Prognostic Factors and Scoring Model for Survival in Metastatic Biliary Tract Cancer
- Affiliations
-
- 1Division of Medical Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 2Cancer Prevention Center, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 3Pancreatobiliary Cancer Clinic, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jeunghc1123@yuhs.ac
- 4Biostatistics Collaboration Unit, Medical Research Center, Yonsei University College of Medicine, Seoul, Korea.
- 5Division of Medical Oncology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 6Songdang Institute for Cancer Research, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2394831
- DOI: http://doi.org/10.4143/crt.2016.538
Abstract
- PURPOSE
Metastatic biliary tract cancer (mBTC) has a dismal prognosis. In this study, an independent dataset of patients with mBTC was used to implement and validate a routine clinico-laboratory parameter-based scoring model for risk group identification.
MATERIALS AND METHODS
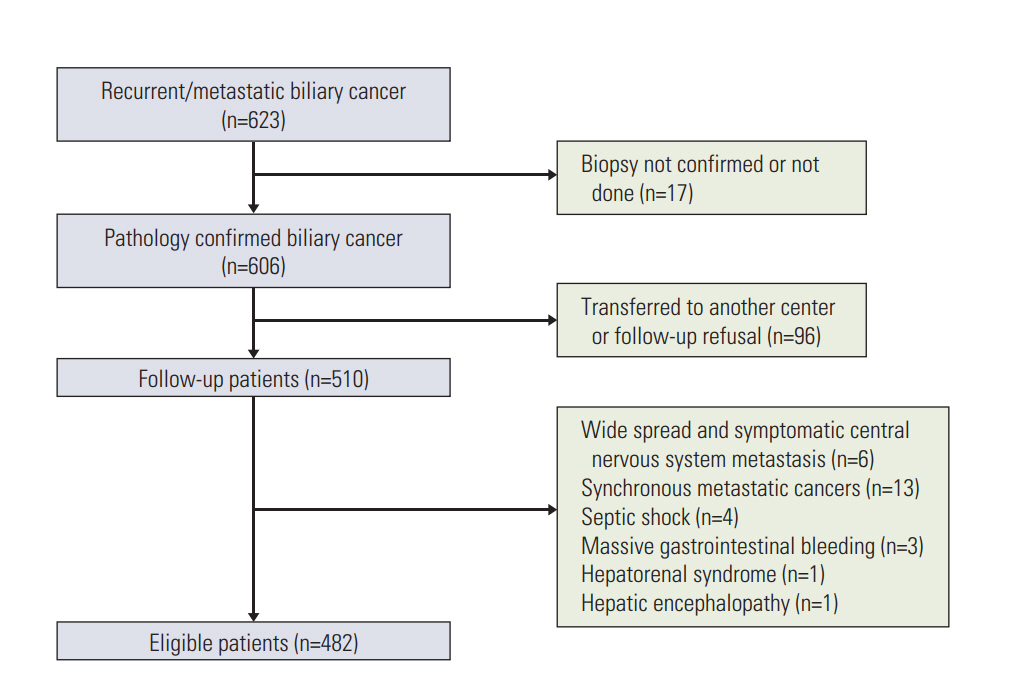
From September 2006 to February 2015, 482 patients with mBTC were assigned randomly (ratio, 7:3) into investigational (n=340) and validation datasets (n=142). The continuous variables were dichotomized using a normal range or the best cutoff values determined using the Contal and O'Quigley statistical methods. Following a Cox's proportional hazard model, the scoring model was derived by summing the rounded chi-square scores for the factors identified by multivariate analysis.
RESULTS
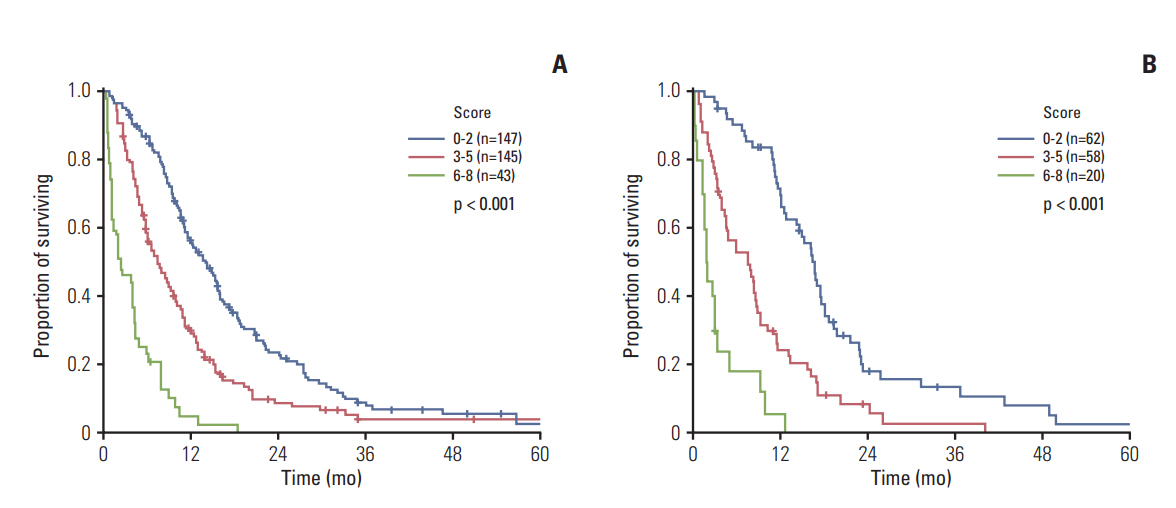
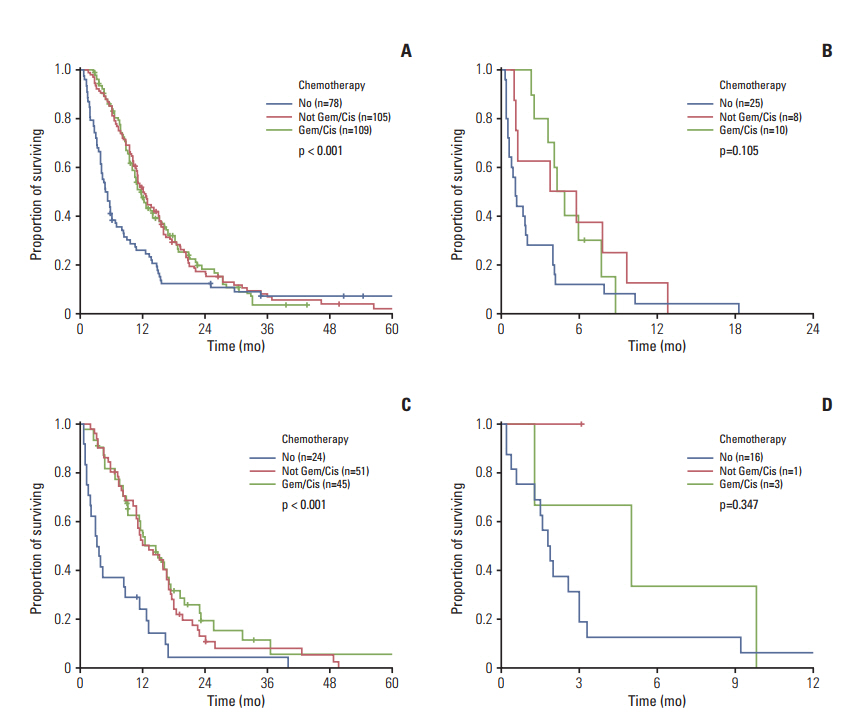
The performance status (Eastern Cooperative Oncology Group 3-4), hypoalbuminemia (< 3.4 mg/dL), carcinoembryonic antigen (≥ 9 ng/mL), neutrophil-to-lymphocyte ratio (≥ 3.0), and carbohydrate antigen 19-9 (≥ 120 U/mL) were identified as independent prognosticators (Harrell's C index, 0.682; integrated area under the curve, 0.653). Survival was clearly correlated with the risk groups (low, intermediate, and high, 14.0, 7.3, and 2.3 months, respectively; p < 0.001). The prognosis was also discriminative in the validation data set (median survival, 16.7, 7.5, and 1.9 months, respectively; p < 0.001). Chemotherapy did not offer any survival benefits for high-risk patients.
CONCLUSION
These proposed prognostic criteria for mBTC can facilitate accurate patient risk stratification and treatment-related decision-making.
MeSH Terms
Figure
Cited by 1 articles
-
A Single-Arm Phase II Study of Nab-Paclitaxel Plus Gemcitabine and Cisplatin for Locally Advanced or Metastatic Biliary Tract Cancer
Ting Liu, Qing Li, Zhen Lin, Chunhua Liu, Wei Pu, Shasha Zeng, Jun Lai, Xuebin Cai, Lisha Zhang, Shuyang Wang, Miao Chen, Wei Cao, Hongfeng Gou, Qing Zhu
Cancer Res Treat. 2024;56(2):602-615. doi: 10.4143/crt.2023.726.
Reference
-
References
1. de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999; 341:1368–78.
Article2. Patel T. Cholangiocarcinoma: controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011; 8:189–200.3. Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002; 37:806–13.
Article4. Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006; 98:873–5.
Article5. Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009; 20:146–59.
Article6. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.
Article7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article8. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article9. McNamara MG, Templeton AJ, Maganti M, Walter T, Horgan AM, McKeever L, et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014; 50:1581–9.
Article10. Peixoto RD, Renouf D, Lim H. A population based analysis of prognostic factors in advanced biliary tract cancer. J Gastrointest Oncol. 2014; 5:428–32.11. Bridgewater J, Lopes A, Wasan H, Malka D, Jensen L, Okusaka T, et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann Oncol. 2016; 27:134–40.
Article12. Iwaku A, Kinoshita A, Onoda H, Fushiya N, Nishino H, Matsushima M, et al. The Glasgow Prognostic Score accurately predicts survival in patients with biliary tract cancer not indicated for surgical resection. Med Oncol. 2014; 31:787.
Article13. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001; 54:774–81.14. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999; 30:253–70.
Article15. Harrell FE Jr, Lee KL, Matchar DB, Reichert TA. Regression models for prognostic prediction: advantages, problems, and suggested solutions. Cancer Treat Rep. 1985; 69:1071–7.16. Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005; 61:92–105.
Article17. Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006; 25:3474–86.
Article18. Proctor MJ, Talwar D, Balmar SM, O'Reilly DS, Foulis AK, Horgan PG, et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010; 103:870–6.
Article19. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010; 9:69.
Article20. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Educational and Clinical Practice Committee; European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003; 22:415–21.
Article21. Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007; 97:1577–82.
Article22. Hsing AW, Gao YT, McGlynn KA, Niwa S, Zhang M, Han TQ, et al. Biliary tract cancer and stones in relation to chronic liver conditions: a population-based study in Shanghai, China. Int J Cancer. 2007; 120:1981–5.
Article23. Hsing AW, Zhang M, Rashid A, McGlynn KA, Wang BS, Niwa S, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer. 2008; 122:1849–53.
Article24. Liu E, Sakoda LC, Gao YT, Rashid A, Shen MC, Wang BS, et al. Aspirin use and risk of biliary tract cancer: a populationbased study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2005; 14:1315–8.
Article25. Saito T, Kuss I, Dworacki G, Gooding W, Johnson JT, Whiteside TL. Spontaneous ex vivo apoptosis of peripheral blood mononuclear cells in patients with head and neck cancer. Clin Cancer Res. 1999; 5:1263–73.26. Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2016; 37:41–52.
Article27. Spolverato G, Maqsood H, Kim Y, Margonis G, Luo T, Ejaz A, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015; 111:868–74.
Article28. Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000; 19:113–32.
Article29. Keam B, Kim DW, Park JH, Lee JO, Kim TM, Lee SH, et al. Nomogram predicting clinical outcomes in non-small cell lung cancer patients treated with epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Res Treat. 2014; 46:323–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Distribution Weighted Prognostic Scoring Model for Node Status in Advanced Rectal Cancer
- Prognostic value of the lymph node metastasis in patients with ampulla of Vater cancer after surgical resection
- A Clinical Analysis of Metastatic Spine Tumors: Analysis of Prognostic Factors and Scoring System for Prognostic Evaluation
- Microbiome and Biliary Tract Cancer
- The Factors Affecting Prognosis in Patients with Metastatic Prostate Cancer after Hormonal Therapy