J Breast Cancer.
2011 Sep;14(3):241-246.
Undifferentiated Pleomorphic Sarcoma of the Male Breast Causing Diagnostic Challenges
- Affiliations
-
- 1Department of Surgery, Catholic University of Daegu College of Medicine, Daegu, Korea. jgbong@cu.ac.kr
- 2Department of Pathology, Catholic University of Daegu College of Medicine, Daegu, Korea.
Abstract
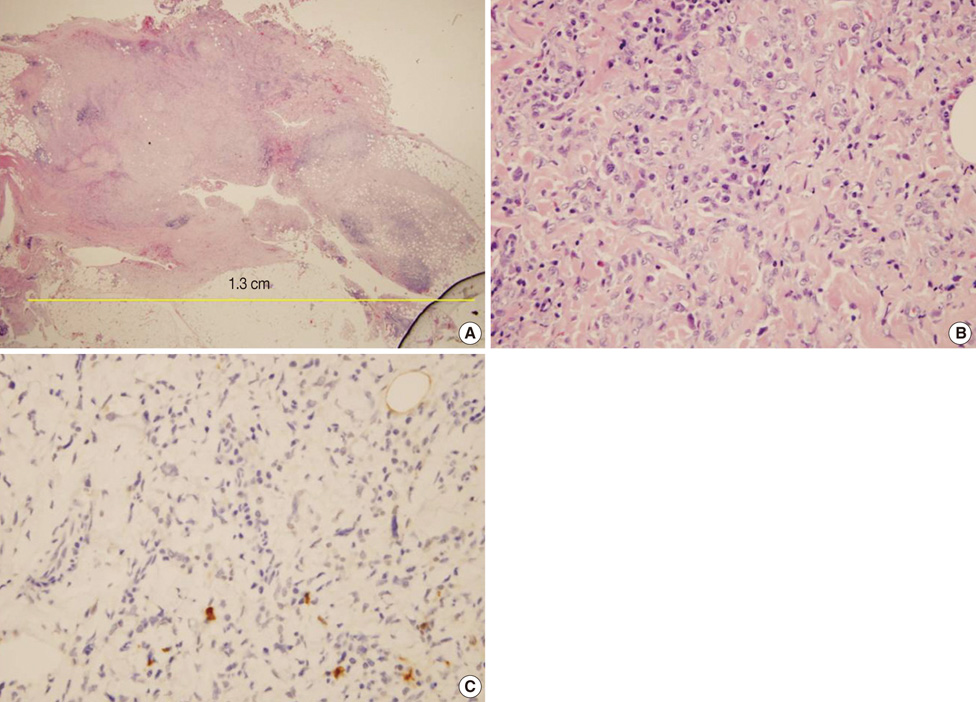
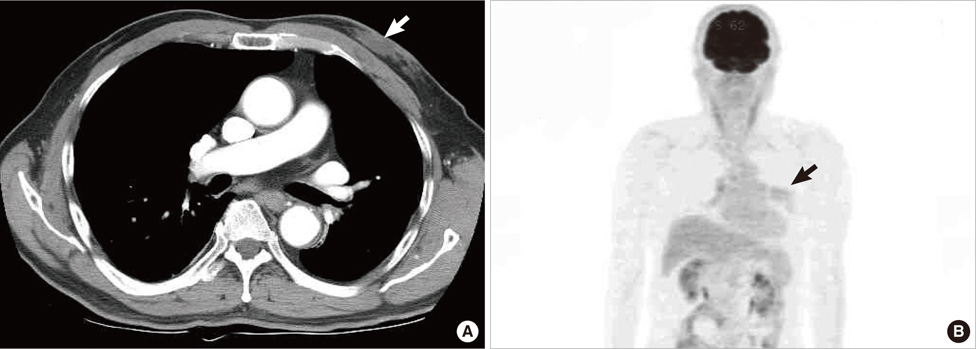
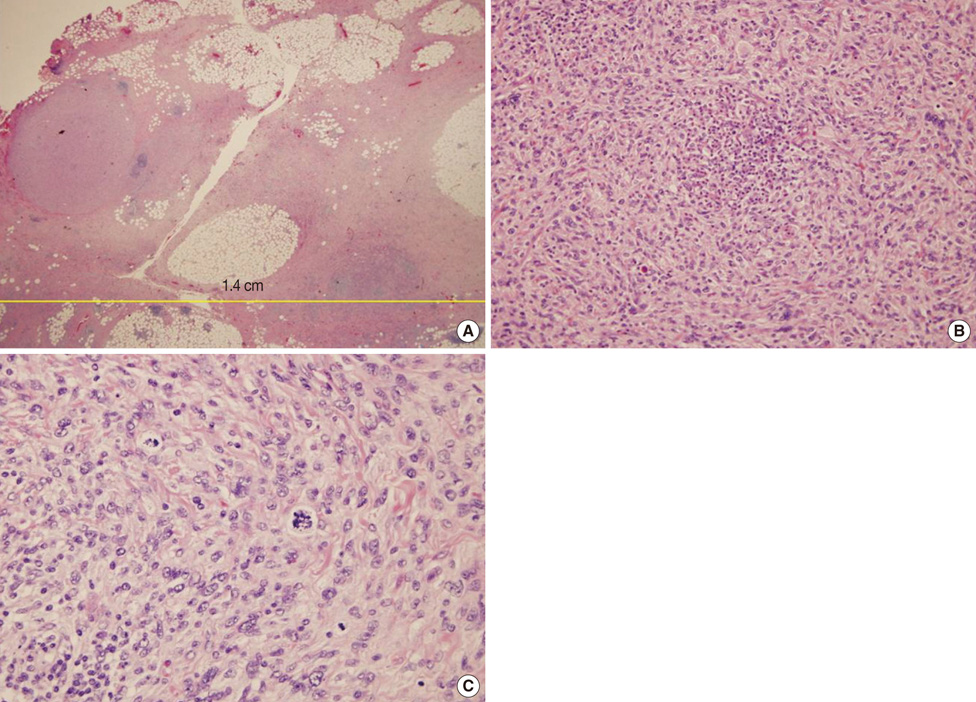
- Undifferentiated pleomorphic sarcoma of the breast are uncommon and often present diagnostic challenges. Herein, we report a case of the undifferentiated pleomorphic sarcoma occurring in the male breast. A 76-year-old man presented with a palpable bean-sized mass in his left breast for two months. Core needle biopsy revealed the presence of atypical cells in a fibrous proliferative lesion, which was removed by wide excision. Based on examination of the excised tumor, the initial pathologic diagnosis was atypical spindle cell lesion with uncertain malignant potential. One year later, the patient returned with a recurrent mass atthe previous surgical site. The mass was again surgically removed using wide excision. Based on histological findings with immunomarkers, the final diagnosis was undifferentiated pleomorphic sarcoma. Undifferentiated pleomorphic sarcoma of the breast can cause genuine diagnostic difficulty and appropriate immunohistochemistry is mandatory for differential diagnosis.
MeSH Terms
Figure
Reference
-
1. Moore MP, Kinne DW. Breast sarcoma. Surg Clin North Am. 1996. 76:383–392.
Article2. Fletcher CD, Unni KK, Mertens F. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Soft Tissue and Bone. 2002. Lyon: IARC Press;9–154.3. Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006. 48:3–12.
Article4. Adem C, Reynolds C, Ingle JN, Nascimento AG. Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer. 2004. 91:237–241.
Article5. Al-Nafussi A. Spindle cell tumours of the breast: practical approach to diagnosis. Histopathology. 1999. 35:1–13.
Article6. Pandey M, Mathew A, Abraham EK, Rajan B. Primary sarcoma of the breast. J Surg Oncol. 2004. 87:121–125.
Article7. Zelger B, Burgdorf WH. Nouri K, editor. Fibrohistiocytic tumors. Skin Cancer. 2007. 1st ed. New York: McGraw-Hill;205–207.8. Jain M, Malhan P. Cytology of soft tissue tumors: pleomorphic sarcoma. J Cytol. 2008. 25:93–96.
Article9. McGowan TS, Cummings BJ, O'Sullivan B, Catton CN, Miller N, Panzarella T. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys. 2000. 46:383–390.
Article10. Shabahang M, Franceschi D, Sundaram M, Castillo MH, Moffat FL, Frank DS, et al. Surgical management of primary breast sarcoma. Am Surg. 2002. 68:673–677.11. Guarino M, Tricomi P, Giordano F, Cristofori E. Sarcomatoid carcinomas: pathological and histopathogenetic considerations. Pathology. 1996. 28:298–305.
Article12. Coffin CM, Dehner LP, Meis-Kindblom JM. Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: an historical review with differential diagnostic considerations. Semin Diagn Pathol. 1998. 15:102–110.13. Magro G. Mammary myofibroblastoma: a tumor with a wide morphologic spectrum. Arch Pathol Lab Med. 2008. 132:1813–1820.
Article14. Magro G, Michal M, Bisceglia M. Benign spindle cell tumors of the mammary stroma: diagnostic criteria, classification, and histogenesis. Pathol Res Pract. 2001. 197:453–466.
Article15. Wargotz ES, Norris HJ, Austin RM, Enzinger FM. Fibromatosis of the breast. A clinical and pathological study of 28 cases. Am J Surg Pathol. 1987. 11:38–45.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Pleomorphic Dermal Sarcoma Showing Characteristics of Myxoinflammatory Fibroblastic Sarcoma
- Cutaneous Metastatic Undifferentiated Pleomorphic Sarcoma from a Mediastinal Sarcoma
- Primary Undifferentiated High-Grade Pleomorphic Sarcoma in the Perihepatic Space: A Report of a Case
- Sinonasal Undifferentiated Pleomorphic Sarcoma in Five Patient Cases
- Undifferentiated Pleomorphic Sarcoma of the Thoracic Aorta: A Case Report