J Korean Orthop Assoc.
2017 Oct;52(5):435-441. 10.4055/jkoa.2017.52.5.435.
Locking Mechanism Failure after Distal Femoral Reconstruction with Modular Universal Tumor and Revision System (MUTARS) Tumor Prosthesis
- Affiliations
-
- 1Department of Orthopedic Surgery, Korea Cancer Center Hospital, Seoul, Korea. wssongmd@gmail.com
- KMID: 2393516
- DOI: http://doi.org/10.4055/jkoa.2017.52.5.435
Abstract
- PURPOSE
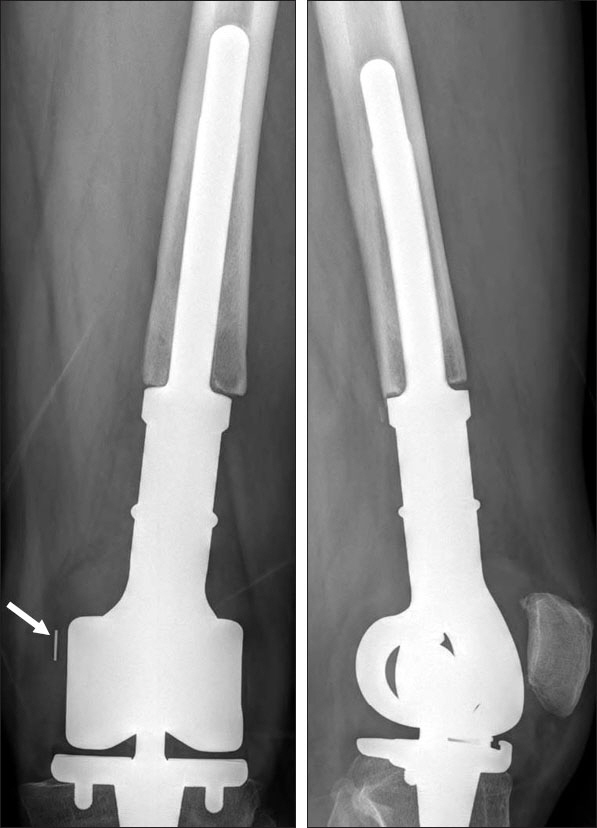
We have used Modular Universal Tumor and Revision System (MUTARS) tumor prosthesis to reconstruct segmental resection defect of the distal femur. The purpose of this study was to evaluate the incidence and pattern of locking mechanism breakage and its correlation with other clinical variables.
MATERIALS AND METHODS
We retrospectively reviewed 94 patients who were followed-up for more than one year after tumor prosthesis replacement (MUTARS) between 2008 and 2013. We examined the incidence and timing of locking mechanism (PEEK-OPTIMA) failure. We also evaluated the clinical characteristics of patients experiencing locking mechanism failure and compared them with those of other patients.
RESULTS
At a mean follow-up of 55 months, we observed locking mechanism failure in 10 of 94 patients (10.6%). The mean age of patients with locking mechanism failure was 29 years (range, 13-54 years); the mean weight and height were 169 cm (range, 151-181 cm) and 67 kg (range, 53-89 kg), respectively. The mean body mass index was 23.5 kg/m² (range, 20.5-29.4 kg/m²). The median time interval between replacement and locking mechanism failure was 26.5 months (range, 12-72 months). The mean body weight of patients with failure was higher than that of patients without failure (p=0.019).
CONCLUSION
The incidence of locking mechanism (PEEK-OPTIMA) failure after distal femoral reconstruction with MUTARS was 11%, and there was a correlation between failure and body weight of patients. Advancements in the design and material of locking mechanisms are warranted to reduce the complication.
Keyword
MeSH Terms
Figure
Reference
-
1. Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007; 89:521–6.2. Chandrasekar CR, Grimer RJ, Carter SR, Tillman RM, Abu-du A, Buckley L. Modular endoprosthetic replacement for tumours of the proximal femur. J Bone Joint Surg Br. 2009; 91:108–12.
Article3. Pala E, Trovarelli G, Calabrò T, Angelini A, Abati CN, Rug-gieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clin Orthop Relat Res. 2015; 473:891–9.
Article4. Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008; 90:1265–71.
Article5. Bus MP, van de Sande MA, Fiocco M, Schaap GR, Bramer JA, Dijkstra PD. What are the long-term results of MUTARS® modular endoprostheses for reconstruction of tumor resection of the distal femur and proximal tibia? Clin Orthop Relat Res. 2017; 475:708–18.6. Capanna R, Morris HG, Campanacci D, Del Ben M, Campa-nacci M. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J Bone Joint Surg Br. 1994; 76:178–86.
Article7. Guo W, Ji T, Yang R, Tang X, Yang Y. Endoprosthetic replacement for primary tumours around the knee: experience from Peking University. J Bone Joint Surg Br. 2008; 90:1084–9.8. Zeegen EN, Aponte-Tinao LA, Hornicek FJ, Gebhardt MC, Mankin HJ. Survivorship analysis of 141 modular metallic endoprostheses at early followup. Clin Orthop Relat Res. 2004; 420:239–50.
Article9. Taylor SJ, Walker PS, Perry JS, Cannon SR, Woledge R. The forces in the distal femur and the knee during walking and other activities measured by telemetry. J Arthroplasty. 1998; 13:428–37.
Article10. Kawai A, Muschler GF, Lane JM, Otis JC, Healey JH. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur. Medium to long-term results. J Bone Joint Surg Am. 1998; 80:636–47.
Article11. McDonald DJ, Capanna R, Gherlinzoni F. . Influence of chemotherapy on perioperative complications in limb salvage surgery for bone tumors. Cancer. 1990; 65:1509–16.
Article12. Berbari EF, Hanssen AD, Duffy MC. . Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998; 27:1247–54.
Article13. Henderson ER, Groundland JS, Pala E. . Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011; 93:418–29.
Article14. Kinkel S, Lehner B, Kleinhans JA, Jakubowitz E, Ewerbeck V, Heisel C. Medium to long-term results after reconstruction of bone defects at the knee with tumor endoprostheses. J Surg Oncol. 2010; 101:166–9.
Article15. Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res. 2005; 438:71–9.16. Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivor-ship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br. 2006; 88:790–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparing Outcomes of Tumor Prosthesis Revision and Locking Plate Fixation in Supracondylar Femoral Periprosthetic Fractures
- The Failure of Polyethylene Lock of MUTARS(R) Distal Femur Tumor Endoprostheses: A Case Report
- Late dissociation of the polyethylene liner from a modular acetabular metal shell after primary total hip arthroplasty: a report of five cases
- Total Hip Revision with Proximal Modular Femoral Stem
- Functional and Radiological Results of Intermediate-term Follow Up in MUTARS(R) Tumor Endoprostheses