Diabetes Metab J.
2017 Aug;41(4):265-274. 10.4093/dmj.2017.41.4.265.
Comparison of Glucose Area Under the Curve Measured Using Minimally Invasive Interstitial Fluid Extraction Technology with Continuous Glucose Monitoring System in Diabetic Patients
- Affiliations
-
- 1Department of Diabetes, Metabolism and Endocrinology, Mie University Graduate School of Medicine, Tsu, Japan. yanoyuta@clin.medic.mie-u.ac.jp
- 2Department of Diabetes and Endocrinology, Mie University Hospital, Tsu, Japan.
- 3Department of Immunology, Mie University Graduate School of Medicine, Tsu, Japan.
- 4Central Research Laboratories, Sysmex Corporation, Kobe, Japan.
- 5Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan.
- 6Department of Gastroenterology and Hepatology, Mie University Graduate School of Medicine, Tsu, Japan.
- KMID: 2392467
- DOI: http://doi.org/10.4093/dmj.2017.41.4.265
Abstract
- BACKGROUND
Continuous glucose monitoring (CGM) is reported to be a useful technique, but difficult or inconvenient for some patients and institutions. We are developing a glucose area under the curve (AUC) monitoring system without blood sampling using a minimally invasive interstitial fluid extraction technology (MIET). Here we evaluated the accuracy of interstitial fluid glucose (IG) AUC measured by MIET in patients with diabetes for an extended time interval and the potency of detecting hyperglycemia using CGM data as a reference.
METHODS
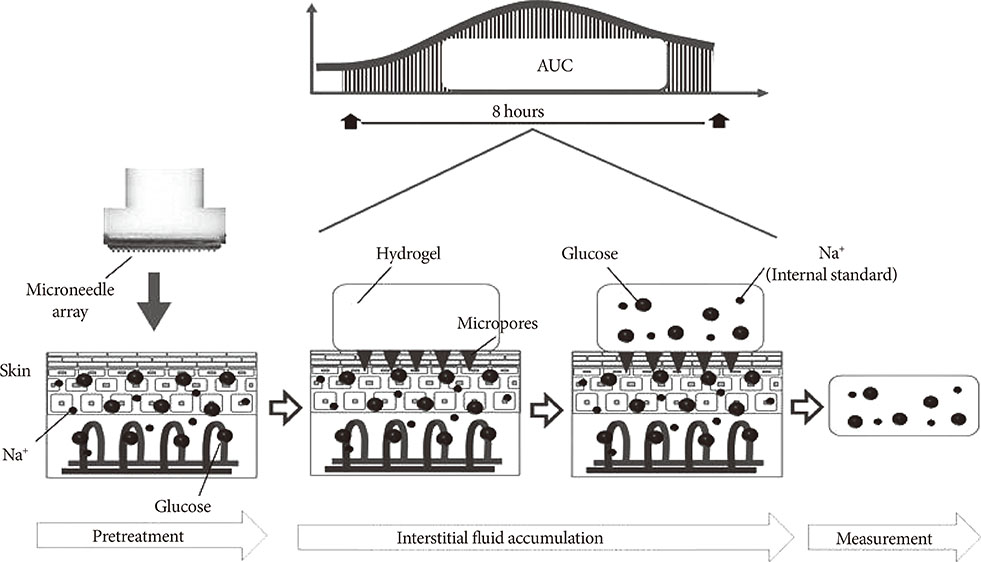
Thirty-eight inpatients with diabetes undergoing CGM were enrolled. MIET comprised a pretreatment step using a plastic microneedle array and glucose accumulation step with a hydrogel patch, which was placed on two sites from 9:00 AM to 5:00 PM or from 10:00 PM to 6:00 AM. IG AUC was calculated by accumulated glucose extracted by hydrogel patches using sodium ion as standard.
RESULTS
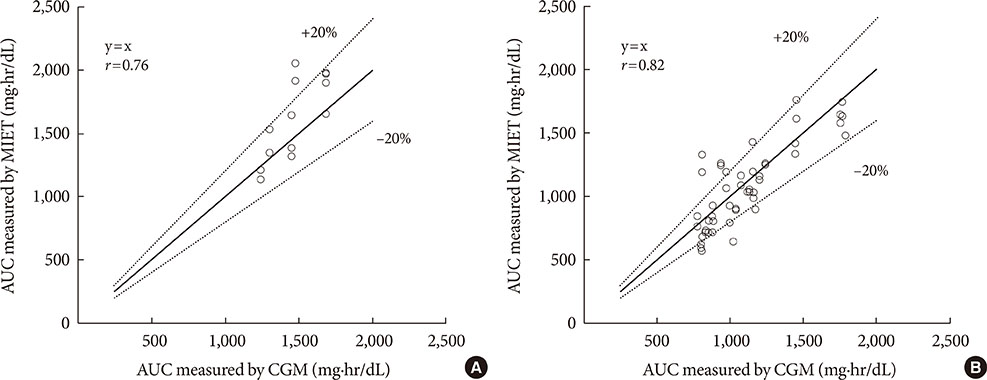
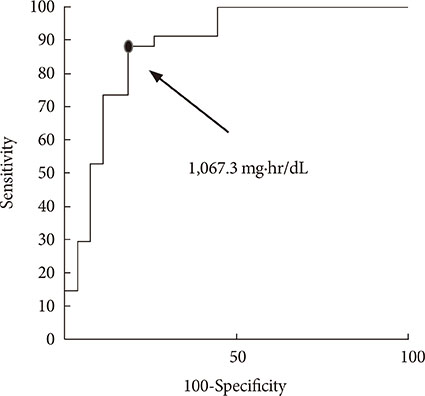
A significant correlation was observed between the predicted AUC by MIET and CGM in daytime (r=0.76) and nighttime (r=0.82). The optimal cutoff for the IG AUC value of MIET to predict hyperglycemia over 200 mg/dL measured by CGM for 8 hours was 1,067.3 mg·hr/dL with 88.2% sensitivity and 81.5% specificity.
CONCLUSION
We showed that 8-hour IG AUC levels using MIET were valuable in estimating the blood glucose AUC without blood sampling. The results also supported the concept of using this technique for evaluating glucose excursion and for screening hyperglycemia during 8 hours in patients with diabetes at any time of day.
Keyword
MeSH Terms
Figure
Reference
-
1. American Diabetes Association. 5. Glycemic targets. Diabetes Care. 2016; 39:Suppl 1. S39–S46.2. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Committee of, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010; 1:212–228.3. Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986.4. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28:103–117.5. DECODE Study Group. European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovas cular diseases? Diabetes Care. 2003; 26:688–696.6. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295:1681–1687.7. Esposito K, Giugliano D, Nappo F, Marfella R. Campanian Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004; 110:214–219.8. Smith-Palmer J, Brandle M, Trevisan R, Orsini Federici M, Liabat S, Valentine W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res Clin Pract. 2014; 105:273–284.9. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016; 18:Suppl 2. S3–S13.10. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016; 388:2254–2263.11. Sato T, Okada S, Hagino K, Asakura Y, Kikkawa Y, Kojima J, Watanabe T, Maekawa Y, Isobe K, Koike R, Nakajima H, Asano K. Measurement of glucose area under the curve using minimally invasive interstitial fluid extraction technology: evaluation of glucose monitoring concepts without blood sampling. Diabetes Technol Ther. 2011; 13:1194–1200.12. Sakaguchi K, Hirota Y, Hashimoto N, Ogawa W, Hamaguchi T, Matsuo T, Miyagawa J, Namba M, Sato T, Okada S, Tomita K, Matsuhisa M, Kaneto H, Kosugi K, Maegawa H, Nakajima H, Kashiwagi A. Evaluation of a minimally invasive system for measuring glucose area under the curve during oral glucose tolerance tests: usefulness of sweat monitoring for precise measurement. J Diabetes Sci Technol. 2013; 7:678–688.13. Ugi S, Maegawa H, Morino K, Nishio Y, Sato T, Okada S, Kikkawa Y, Watanabe T, Nakajima H, Kashiwagi A. Evaluation of a novel glucose area under the curve (AUC) monitoring system: comparison with the AUC by continuous glucose monitoring. Diabetes Metab J. 2016; 40:326–333.14. Ando Y, Ito S, Uemura O, Kato T, Kimura G, Nakao T, Hattori M, Fukagawa M, Horio M, Mitarai T. Japanese Society of Nephrology. CKD clinical practice guidebook. The essence of treatment for CKD patients. Clin Exp Nephrol. 2009; 13:191–248.15. Service FJ. Glucose variability. Diabetes. 2013; 62:1398–1404.16. Wojcicki JM. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm Metab Res. 1995; 27:41–42.17. Schlichtkrull J, Munck O, Jersild M. The m-valve, an index of blood-sugar control in diabetics. Acta Med Scand. 1965; 177:95–102.18. Sakamoto K, Kubo F, Yoshiuchi K, Ono A, Sato T, Tomita K, Sakaguchi K, Matsuhisa M, Kaneto H, Maegawa H, Nakajima H, Kashiwagi A, Kosugi K. Usefulness of a novel system for measuring glucose area under the curve while screening for glucose intolerance in outpatients. J Diabetes Investig. 2013; 4:552–559.19. Kuranuki S, Sato T, Okada S, Hosoya S, Seko A, Sugihara K, Nakamura T. Evaluation of postprandial glucose excursion using a novel minimally invasive glucose area-under-the-curve monitoring system. J Healthc Eng. 2013; 4:529–540.20. Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001; 24:1858–1862.21. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008; 31:1473–1478.22. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, Lernmark A, Metzger BE, Nathan DM. National Academy of Clinical Biochemistry. Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011; 34:1419–1423.23. Suwa T, Ohta A, Matsui T, Koganei R, Kato H, Kawata T, Sada Y, Ishii S, Kondo A, Murakami K, Katabami T, Tanaka Y. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J. 2010; 57:135–140.24. Ando K, Nishimura R, Tsujino D, Seo C, Utsunomiya K. 24-Hour glycemic variations in drug-naive patients with type 2 diabetes: a continuous glucose monitoring (CGM)-based study. PLoS One. 2013; 8(7):e71102.25. Sakamoto M, Nishimura R, Irako T, Tsujino D, Ando K, Utsunomiya K. Comparison of vildagliptin twice daily vs. sitagliptin once daily using continuous glucose monitoring (CGM): crossover pilot study (J-VICTORIA study). Cardiovasc Diabetol. 2012; 11:92.26. Chan CL, Pyle L, Newnes L, Nadeau KJ, Zeitler PS, Kelsey MM. Continuous glucose monitoring and its relationship to hemoglobin A1c and oral glucose tolerance testing in obese and prediabetic youth. J Clin Endocrinol Metab. 2015; 100:902–910.27. Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015; 39:273–282.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of a Novel Glucose Area Under the Curve (AUC) Monitoring System: Comparison with the AUC by Continuous Glucose Monitoring
- Application of Continuous Glucose Monitoring System (CGMS) and Patient Education
- Correlations of Glucose Levels in Interstitial Fluid Estimated by Continuous Glucose Monitoring Systems and Venous Plasma
- New Technology for Type 1 Diabetes
- Glucose Management Using Continuous Glucose Monitors