J Vet Sci.
2012 Dec;13(4):363-369.
A novel mycotoxin purification system using magnetic nanoparticles for the recovery of aflatoxin B1 and zearalenone from feed
- Affiliations
-
- 1Animal, Plant and Fisheries Quarantine and Inspection Agency, Anyang 480-757, Korea. virusmania@korea.kr
- 2Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-742, Korea.
Abstract
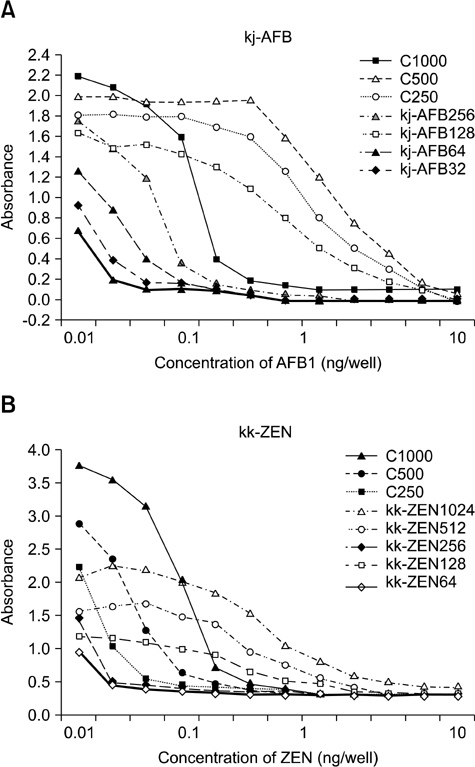
- In this study, we developed a novel tool for purifying two mycotoxins, aflatoxin B1 (AFB1) and zearalenone (ZEN), in feed. This system utilized monoclonal antibodies (mAbs) against AFB1 and ZEN, and magnetic nanoparticles (MNPs). Among ten MNPs with different diameters and functional groups, a 100-nm diameter MNP (fMA) conjugated to an amine group (-NH2) was found to be optimum for coupling with mAbs. The optimal mAb concentrations for coupling to the fMA along with mycotoxin purification capacities of the fMA-mAb conjugates (fMA-AFB1 and fMA-ZEN) were determined. A comparison of mean recovery rates (from corn and product X feed) between the fMA-mAb conjugates and immunoaffinity columns (IAC-AFB1 and IAC-ZEN) showed that the rate for fMA-AFB1 (90~92% and 81~88%) was higher (p > 0.05) than that of IAC-AFB1 (81~84% and 72~78%) for AFB1 (5, 10, 15 ng/mL), and the rate for fMA-ZEN (99~100% and 92~94%) was significantly higher (p < 0.01) than that of IAC-ZEN (86~88% and 81~88%) for ZEN (10, 25, 50 ng/mL) except at a concentration of 10 ng/mL, demonstrating the remarkable purification efficiency of the novel fMA-mAb method. Additionally, mycotoxin purification was much faster using our novel method (approx. 5 min) than the IAC-based technique (> 30 min). This study suggests that the novel purification system we developed would be a useful tool for monitoring and regulating mycotoxin contamination in feed, and replace IAC methods.
MeSH Terms
Figure
Reference
-
1. Ban C, Ramakrishnan B, Sundaralingam M. Crystal structure of the highly distorted chimeric decamer r(C)d(CGGCGCCG)r(G)·spermine complex--spermine binding to phosphate only and minor groove tertiary base-pairing. Nucleic Acids Res. 1994. 22:5466–5476.
Article2. Caldwell RW, Tuite J, Stob M, Baldwin R. Zearalenone production by Fusarium species. Appl Microbiol. 1970. 20:31–34.3. Castegnaro M, Tozlovanu M, Wild C, Molinié A, Sylla A, Pfohl-Leszkowicz A. Advantages and drawbacks of immunoaffinity columns in analysis of mycotoxins in food. Mol Nutr Food Res. 2006. 50:480–487.
Article4. Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006. 107:459–466.
Article5. Duan HL, Shen ZQ, Wang XW, Chao FH, Li JW. Preparation of immunomagnetic iron-dextran nanoparticles and application in rapid isolation of E. coli O157:H7 from foods. World J Gastroenterol. 2005. 11:3660–3664.
Article6. Gao M, Deng C, Fan Z, Yao N, Xu X, Yang P, Zhang X. A simple pathway to the synthesis of magnetic nanoparticles with immobilized metal ions for the fast removal of microcystins in water. Small. 2007. 3:1714–1717.
Article7. Hibi K, Mitsubayashi K, Fukuda H, Ushio H, Hayashi T, Ren H, Endo H. Rapid direct determination using combined separation by prepared immunomagnetic and flow cytometry of Flavobacterium psychrophilum. Biosens Bioelectron. 2007. 22:1916–1919.
Article8. Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001. 167:101–134.
Article9. Jain KK. Applications of nanobiotechnology in clinical diagnostics. Clin Chem. 2007. 53:2002–2009.
Article10. Kallela K, Ettala E. The oestrogenic Fusarium toxin (zearalenone) in hay as a cause of early abortions in the cow. Nord Vet Med. 1984. 36:305–309.11. Krska R, Schubert-Ullrich P, Molinelli A, Sulyok M, MacDonald S, Crews C. Mycotoxin analysis: an update. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008. 25:152–163.
Article12. Lugauskas A, Raila A, Zvicevičius E, Railienė M, Novošinskas H. Factors determining accumulation of mycotoxin producers in cereal grain during harvesting. Ann Agric Environ Med. 2007. 14:173–186.13. Malekinejad H, Schoevers EJ, Daemen IJJM, Zijlstra C, Colenbrander B, Fink-Gremmels J, Roelen BAJ. Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biol Reprod. 2007. 77:840–847.
Article14. Martins ML, Martins HM, Bernardo F. Aflatoxins in spices marketed in Portugal. Food Addit Contam. 2001. 18:315–319.
Article15. McBain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008. 3:169–180.16. Ryu JC, Yang JS, Song YS, Kwon OS, Park J, Chang IM. Survey of natural occurrence of trichothecene mycotoxins and zearalenone in Korean cereals harvested in 1992 using gas chromatography/mass spectrometry. Food Addit Contam. 1996. 13:333–341.
Article17. Solfrizzo M, De Girolamo A, Visconti A. Determination of fumonisins B1 and B2 in cornflakes by high performance liquid chromatography and immunoaffinity clean-up. Food Addit Contam. 2001. 18:227–235.
Article18. Thongrussamee T, Kuzmina NS, Shim WB, Jiratpong T, Eremin SA, Intrasook J, Chung DH. Monoclonal-based enzyme-linked immunosorbent assay for the detection of zearalenone in cereals. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008. 25:997–1006.
Article19. Turner NW, Subrahmanyam S, Piletsky SA. Analytical methods for determination of mycotoxins: a review. Anal Chim Acta. 2009. 632:168–180.
Article20. Visconti A, Pascale M. Determination of zearalenone in corn by means of immunoaffinity clean-up and high-performance liquid chromatography with fluorescence detection. J Chromatogr A. 1998. 815:133–140.
Article21. Yang H, Qu L, Wimbrow AN, Jiang X, Sun Y. Rapid detection of Listeria monocytogenes by nanoparticle-based immunomagnetic separation and real-time PCR. Int J Food Microbiol. 2007. 118:132–138.
Article22. Yantasee W, Warner CL, Sangvanich T, Addleman RS, Carter TG, Wiacek RJ, Fryxell GE, Timchalk C, Warner MG. Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ Sci Technol. 2007. 41:5114–5119.
Article23. Yin YN, Yan LY, Jiang JH, Ma ZH. Biological control of aflatoxin contamination of crops. J Zhejiang Univ Sci B. 2008. 9:787–792.
Article24. Zmudzki J, Wiśniewska-Dmytrow H. Limits and regulations for mycotoxins in food and feed. Pol J Vet Sci. 2004. 7:211–216.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of aflatoxin producing Aspergillus flavus from animal feed in Karnataka, India.
- The measurement of aflatoxin B in urine of some Korean
- Reduction in Binding of Aflatoxin B1 to Rat Liver DNA by Vitamin A and E Pretreatment
- Fine Structural Changes and Autoradiographic Studies of Rat Liver Cells Induced by Aflatoxin B1 and G1
- A study on the analysis of aflatoxin B in human sera by ELISA