Chonnam Med J.
2017 Sep;53(3):216-222. 10.4068/cmj.2017.53.3.216.
Factors Associated with C-peptide Levels after Diagnosis in Children with Type 1 Diabetes Mellitus
- Affiliations
-
- 1Department of Pediatrics, Chonbuk National University Medical School, Jeonju, Korea. leedy@jbnu.ac.kr
- 2Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2390436
- DOI: http://doi.org/10.4068/cmj.2017.53.3.216
Abstract
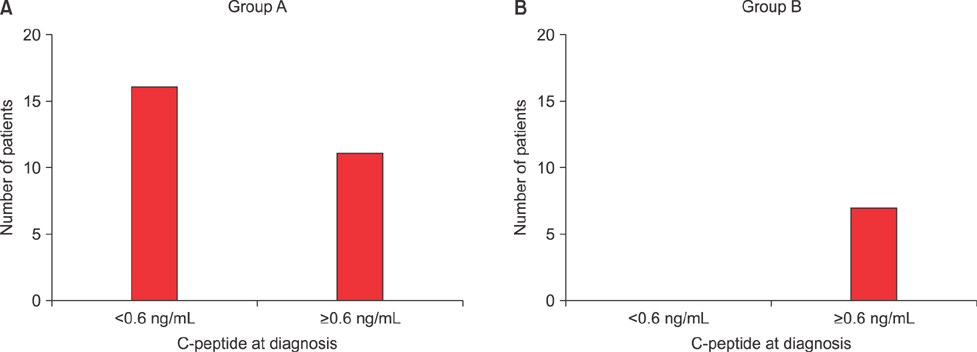
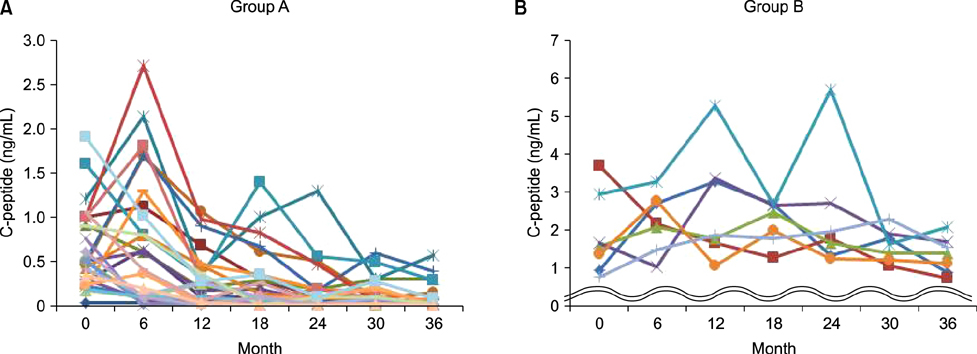
- C-peptide is the best indicator of endogenous insulin secretion in patients with diabetes. This study investigated the relationship between C-peptide levels and clinical/laboratory parameters of children with type 1 diabetes mellitus (T1DM), as measured at 6-month intervals after diagnosis. We retrospectively reviewed the data of 34 children with newly diagnosed T1DM. The study subjects were subdivided into a rapid progression group with C-peptide levels <0.6 ng/mL at 36 months (n=27; Group A) and a slow progression group with C-peptide levels >0.6 ng/mL at 36 months (n=7; Group B). Patients in Group A had a younger mean age at diagnosis (A: 9±4.3 years vs. B: 13.6±3.6 years; p=0.013) and lower body mass index (BMI) (A: 15.5±2.5 kg/m² vs. B: 18.7±3.3 kg/m²; p=0.035). There were fewer asymptomatic patients with glucosuria in Group A, with these patients showing more severe symptoms, such as diabetic ketoacidosis (p=0.035), than those in Group B. Group A also had lower initial C-peptide levels (A: 0.5±0.46 ng/mL vs. B: 1.87±1.08 ng/mL; p=0.001). There were no significant intergroup differences in sex, family history, baseline hemoglobin A1c (HbA1c), potential of hydrogen (pH), autoantibodies or serum insulin. Simple correlation analyses showed that C-peptide levels were correlated with age and BMI, but not with pH, insulin, or HbA1c. Younger patients, who had a lower BMI, significant symptoms with complications, and/or a low initial C-peptide level, tended to show a rapid rate of decrease in C-peptide levels. Early intensive insulin therapy to preserve beta-cell function should be considered in these groups.
MeSH Terms
Figure
Reference
-
1. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013; 30:803–817.
Article2. Ludvigsson J, Carlsson A, Forsander G, Ivarsson S, Kockum I, Lernmark A, et al. C-peptide in the classification of diabetes in children and adolescents. Pediatr Diabetes. 2012; 13:45–50.
Article3. Steck AK, Dong F, Waugh K, Frohnert BI, Yu L, Norris JM, et al. Predictors of slow progression to diabetes in children with multiple islet autoantibodies. J Autoimmun. 2016; 72:113–117.
Article4. Achenbach P, Hummel M, Thümer L, Boerschmann H, Höfelmann D, Ziegler AG. Characteristics of rapid vs slow progression to type 1 diabetes in multiple islet autoantibody-positive children. Diabetologia. 2013; 56:1615–1622.
Article5. Redondo MJ, Rodriguez LM, Escalante M, O'Brian Smith E, Balasubramanyam A, Haymond MW. Beta cell function and BMI in ethnically diverse children with newly diagnosed autoimmune type 1 diabetes. Pediatr Diabetes. 2012; 13:564–571.
Article6. Soltesz G, Patterson CC, Dahlquist G. EURODIAB Study Group. Worldwide childhood type 1 diabetes incidence--what can we learn from epidemiology. Pediatr Diabetes. 2007; 8:Suppl 6. 6–14.
Article7. Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. J Clin Endocrinol Metab. 1987; 65:30–36.8. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998; 128:517–523.9. Borg H, Arnqvist HJ, Björk E, Bolinder J, Eriksson JW, Nyström L, et al. Evaluation of the new ADA and WHO criteria for classification of diabetes mellitus in young adult people (15-34 yrs) in the Diabetes Incidence Study in Sweden (DISS). Diabetologia. 2003; 46:173–181.
Article10. Besser RE. Determination of C-peptide in children: when is it useful? Pediatr Endocrinol Rev. 2013; 10:494–502.11. Aanstoot HJ, Anderson BJ, Daneman D, Danne T, Donaghue K, Kaufman F, et al. The global burden of youth diabetes: perspectives and potential. Pediatr Diabetes. 2007; 8:Suppl 8. 1–44.12. Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2009; 10:Suppl 12. 3–12.
Article13. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003; 26:832–836.
Article14. Sjöberg S, Gunnarsson R, Gjötterberg M, Lefvert AK, Persson A, Ostman J. Residual insulin production, glycaemic control and prevalence of microvascular lesions and polyneuropathy in long-term type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987; 30:208–213.
Article15. Panero F, Novelli G, Zucco C, Fornengo P, Perotto M, Segre O, et al. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care. 2009; 32:301–305.
Article16. Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004; 53:250–264.
Article17. Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989; 320:550–554.
Article18. Yu HW, Lee YJ, Cho WI, Lee YA, Shin CH, Yang SW. Preserved C-peptide levels in overweight or obese compared with underweight children upon diagnosis of type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab. 2015; 20:92–97.
Article19. Williams GM, Long AE, Wilson IV, Aitken RJ, Wyatt RC, McDonald TJ, et al. Beta cell function and ongoing autoimmunity in long-standing, childhood onset type 1 diabetes. Diabetologia. 2016; 59:2722–2726.
Article20. Davis AK, DuBose SN, Haller MJ, Miller KM, DiMeglio LA, Bethin KE, et al. Prevalence of detectable C-Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015; 38:476–481.
Article21. Urakami T, Miyamoto Y, Fujita H, Kitagawa T. Type 1 (insulin-dependent) diabetes in Japanese children is not a uniform disease. Diabetologia. 1989; 32:312–315.
Article22. Wang Z, Xie Z, Lu Q, Chang C, Zhou Z. Beyond genetics: what causes type 1 diabetes. Clin Rev Allergy Immunol. 2017; 52:273–286.
Article23. Urakami T, Miyamoto Y, Matsunaga H, Owada M, Kitagawa T. Serial changes in the prevalence of islet cell antibodies and islet cell antibody titer in children with IDDM of abrupt or slow onset. Diabetes Care. 1995; 18:1095–1099.
Article24. Stenström G, Gottsäter A, Bakhtadze E, Berger B, Sundkvist G. Latent autoimmune diabetes in adults: definition, prevalence, beta-cell function, and treatment. Diabetes. 2005; 54:Suppl 2. S68–S72.25. Greenbaum CJ, Anderson AM, Dolan LM, Mayer-Davis EJ, Dabelea D, Imperatore G, et al. Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care. 2009; 32:1839–1844.
Article26. Sochett EB, Daneman D, Clarson C, Ehrlich RM. Factors affecting and patterns of residual insulin secretion during the first year of type 1 (insulin-dependent) diabetes mellitus in children. Diabetologia. 1987; 30:453–459.
Article27. Sørensen JS, Johannesen J, Pociot F, Kristensen K, Thomsen J, Hertel NT, et al. Residual XMLLink_XYZ-Cell function 3-6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care. 2013; 36:3454–3459.
Article28. Tatovic D, Luzio S, Dunseath G, Liu Y, Alhadj Ali M, Peakman M, et al. Stimulated urine C-peptide creatinine ratio vs serum C-peptide level for monitoring of β-cell function in the first year after diagnosis of Type 1 diabetes. Diabet Med. 2016; 33:1564–1568.
Article29. Ludvigsson J, Heding LG. C-peptide in children with juvenile diabetes. A preliminary report. Diabetologia. 1976; 12:627–630.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Types and Characteristics of Diabetes Mellitus in Korean Children
- The Clinical Measures Associated with C-peptide Decline in Patients with Type 1 Diabetes over 15 Years
- Diagnosis and treatment of pediatric type 2 diabetes mellitus
- Factors Related to Blood Intact Incretin Levels in Patients with Type 2 Diabetes Mellitus
- Factors Influencing the Onset of Honeymoon Period in Children with Type I Diabetes Mellitus