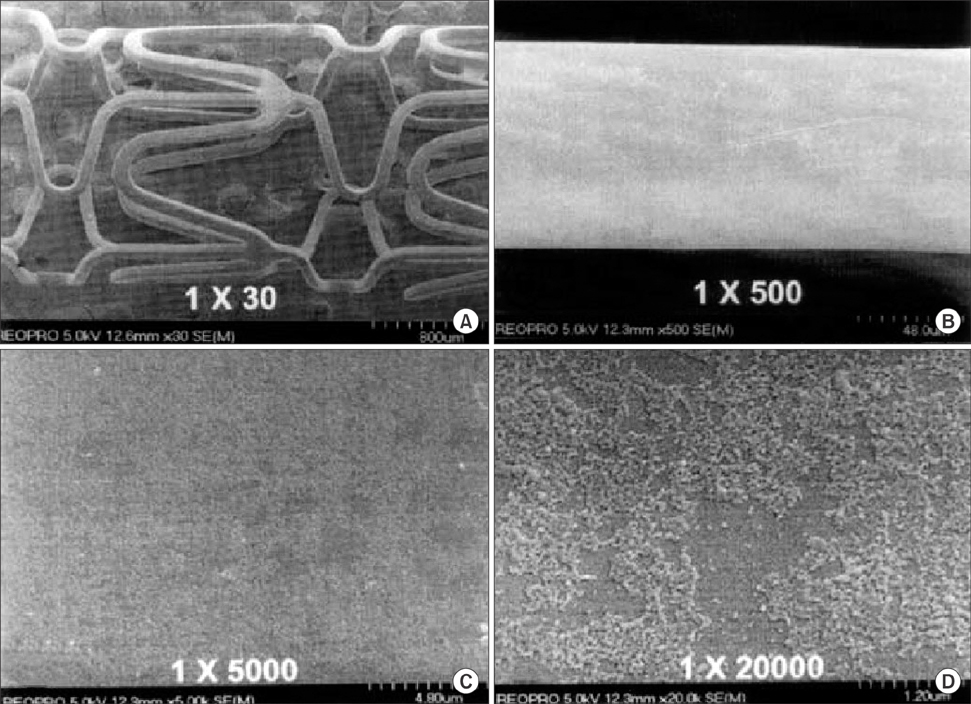
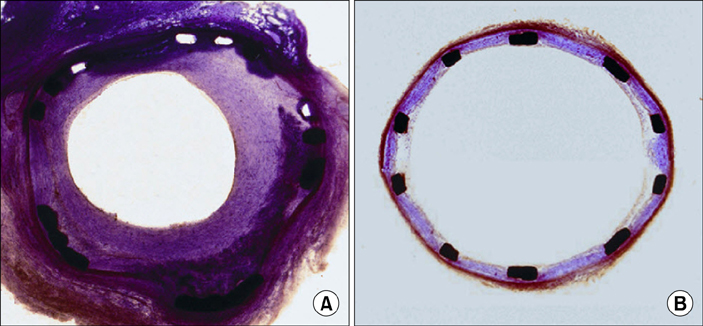
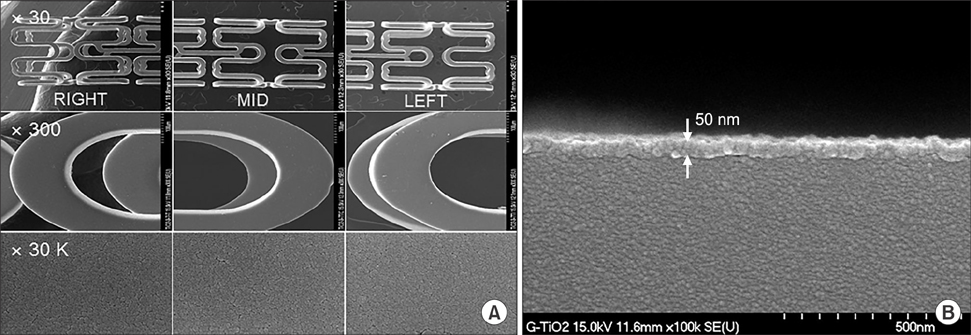
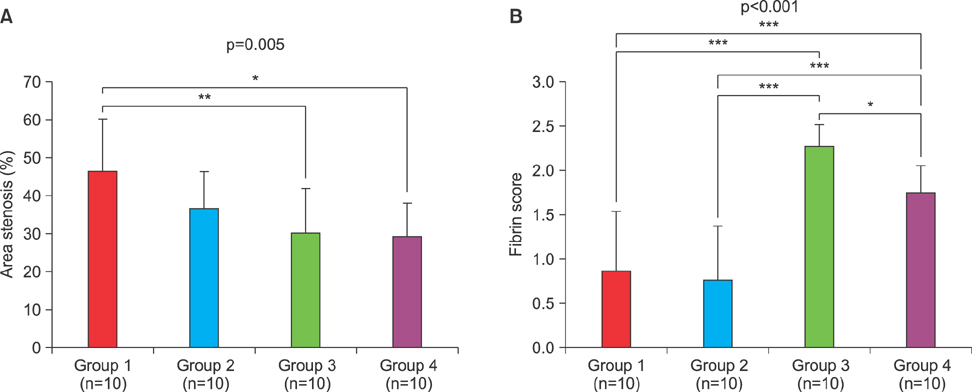
Chonnam Med J.
2017 Sep;53(3):187-195. 10.4068/cmj.2017.53.3.187.
Development of Novel Drug-Eluting Stents for Acute Myocardial Infarction
- Affiliations
-
- 1Department of Cardiovascular Medicine, Chonnam National University Hospital, Gwangju, Korea. myungho@chollian.net
- KMID: 2390432
- DOI: http://doi.org/10.4068/cmj.2017.53.3.187
Abstract
- Delayed arterial healing at culprit sites after drug-eluting stent (DES) placement for acute myocardial infarction (AMI) is associated with increased risk of late stent thrombosis. The Korea Acute Myocardial Infarction Registry was established in commemoration of the 50(th) anniversary of Korea Circulation Society. Between November 2005 and December 2016, more than 62,000 patients were registered from 50 primary percutaneous coronary intervention (PCI) centers in Korea. DES in AMI may be safe and effective, however, there is concern about increased stent thrombosis after DES implantation in AMI patients, requiring longer-term dual anti-platelet therapy to reduce the risk of late stent thrombosis. Device innovation is needed to overcome issues such as stent thrombosis and restenosis by using new coating materials with biocompatible polymers, different coating methods using non-polymer techniques, bioabsorbable stents and pro-healing stents. In this review article, we describe the current usage of DES in AMI in Korea and introduce novel DES uses in development for patients with AMI. We have developed many types of DES in our research laboratory. Abciximab-coated stents inhibited platelet thrombi and restenosis. Furthermore, anti-oxidants (carvedilol, probucol and alpha-lipoic acid) were used for stent coating. Currently we are developing novel DESs using polymer-free and natural binding techniques, peptide coating stents, gene-and-drug delivery, bioabsorbable stents using 3D printing, endothelial progenitor cell capturing stents to promote reendothelialization and reduce stent thrombosis. New DESs in development may be safe and effective in preventing late stent thrombosis and restenosis in patients with AMI.
MeSH Terms
Figure
Cited by 1 articles
-
Preclinical Evaluation of a Novel Polymer-free Everolimus-eluting Stent in a Mid-term Porcine Coronary Restenosis Model
Kyung Hoon Cho, Myung Ho Jeong, Dae Sung Park, Moonki Kim, JungHa Kim, Jun-Kyu Park, Xiongyi Han, Dae Young Hyun, Min Chul Kim, Doo Sun Sim, Young Joon Hong, Ju Han Kim, Youngkeun Ahn
J Korean Med Sci. 2021;36(40):e259. doi: 10.3346/jkms.2021.36.e259.
Reference
-
1. Lee KH, Jeong MH, Kim HM, Ahn Y, Kim JH, Chae SC, et al. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2011; 58:1664–1671.
Article2. Kim JH, Chae SC, Oh DJ, Kim HS, Kim YJ, Ahn Y, et al. Multicenter cohort study of acute myocardial infarction in Korea - Interim analysis of the Korea acute myocardial infarction registry-national institutes of health registry. Circ J. 2016; 80:1427–1436.
Article3. Kook HY, Jeong MH, Oh S, Yoo SH, Kim EJ, Ahn Y, et al. Current trend of acute myocardial infarction in Korea (from the Korea Acute Myocardial Infarction Registry from 2006 to 2013). Am J Cardiol. 2014; 114:1817–1822.
Article4. Lee SR, Jeong MH, Ahn YK, Chae SC, Hur SH, Kim YJ, et al. Clinical safety of drug-eluting stents in the Korea acute myocardial infarction registry. Circ J. 2008; 72:392–398.
Article5. Hong YJ, Jeong MH, Ahn Y, Kang JC. The efficacy and safety of drug-eluting stents in patients with acute myocardial infarction: results from Korea Acute Myocardial Infarction (KAMIR). Int J Cardiol. 2013; 163:1–4.
Article6. Hong YJ, Jeong MH, Ahn Y, Sim DS, Chung JW, Cho JS, et al. Plaque prolapse after stent implantation in patients with acute myocardial infarction: an intravascular ultrasound analysis. JACC Cardiovasc Imaging. 2008; 1:489–497.
Article7. Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, et al. Plaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patients. JACC Cardiovasc Imaging. 2009; 2:339–349.
Article8. Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis. Eur Heart J. 2011; 32:2059–2066.
Article9. Hong YJ, Jeong MH, Choi YH, Ma EH, Ko JS, Lee MG, et al. Effect of renal function on ultrasonic coronary plaque characteristics in patients with acute myocardial infarction. Am J Cardiol. 2010; 105:936–942.
Article10. Hong YJ, Jeong MH, Choi YH, Park SY, Rhew SH, Jeong HC, et al. Clinical, angiographic, and intravascular ultrasound predictors of early stent thrombosis in patients with acute myocardial infarction. Int J Cardiol. 2013; 168:1674–1675.
Article11. Lim SY, Jeong MH, Hong SJ, Lim DS, Moon JY, Hong YJ, et al. Inflammation and delayed endothelization with overlapping drug-eluting stents in a porcine model of in-stent restenosis. Circ J. 2008; 72:463–468.
Article12. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007; 115:2435–2441.
Article13. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006; 48:193–202.14. Cho JY, Ahn Y, Jeong MH. A bumpy and winding but right path to domestic drug-eluting coronary stents. Korean Circ J. 2013; 43:645–654.
Article15. Bae IH, Lim KS, Park JK, Park DS, Lee SY, Jang EJ, et al. Mechanical behavior and in vivo properties of newly designed bare metal stent for enhanced flexibility. J Ind Eng Chem. 2015; 21:1295–1300.
Article16. Ahn YK, Jeong MH, Kim JW, Kim SH, Cho JH, Cho JG, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999; 48:324–330.
Article17. Hong YJ, Jeong MH, Lee SR, Hong SN, Kim KH, Park HW, et al. Anti-inflammatory effect of abciximab-coated stent in a porcine coronary restenosis model. J Korean Med Sci. 2007; 22:802–809.
Article18. Hong YJ, Jeong MH, Kim W, Lim SY, Lee SH, Hong SN, et al. Effect of abciximab-coated stent on in-stent intimal hyperplasia in human coronary arteries. Am J Cardiol. 2004; 94:1050–1054.
Article19. Kim W, Jeong MH, Kim KH, Sohn IS, Hong YJ, Park HW, et al. The clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (abciximab: ReoPro)-coated stent in acute myocardial infarction. J Am Coll Cardiol. 2006; 47:933–938.
Article20. Kim W, Jeong MH, Cha KS, Lee SH, Lim JH, Kim HG, et al. The effect of the probucol-loaded biodivYsioTM DD stent on inhibition of neointimal proliferation in porcine coronary stent restenosis model. Korean Circ J. 2003; 33:1028–1035.
Article21. Kim W, Jeong MH, Cha KS, Hyun DW, Hur SH, Kim KB, et al. Effect of anti-oxidant (carvedilol and probucol) loaded stents in a porcine coronary restenosis model. Circ J. 2005; 69:101–106.
Article22. Lim SY, Bae EH, Jeong MH, Kim JH, Hong YJ, Sim DS, et al. Effect of alpha lipoic acid in a porcine in-stent restenosis model. J Cardiol. 2009; 54:375–385.23. Kim HK, Hong YJ, Jeong MH, Kim W, Kim SS, Ko JS, et al. Two-year clinical outcome after carvedilol-loaded stent implantation in patients with coronary artery disease. Korean J Intern Med. 2011; 26:41–46.
Article24. Song SJ, Park YJ, Park J, Cho MD, Kim JH, Jeong MH, et al. Preparation of a drug-eluting stent using a TiO2 film deposited by plasma enhanced chemical vapour deposition as a drug-combining matrix. J Mater Chem. 2010; 20:4792–4801.
Article25. Song SJ, Kim KS, Park YJ, Jeong MH, Ko YM, Cho DL. Preparation of a dual-drug-eluting stent by grafting of ALA with abciximab on a bare metal stent. J Mater Chem. 2009; 19:8135–8141.
Article26. Lim KS, Jeong MH, Bae IH, Park JK, Park DS, Kim JM, et al. Effect of polymer-free TiO2 stent coated with abciximab or alpha lipoic acid in porcine coronary restenosis model. J Cardiol. 2014; 64:409–418.
Article27. Song SJ, Jung KW, Park YJ, Park J, Cho MD, Jeong MH, et al. Nitrogen-doped TiO2 films as drug-binding matrices for the preparation of drug-eluting stents. J Mater Chem. 2011; 21:8169–8177.
Article28. Sim DS, Jeong MH, Park DS, Kim JH, Lim KS, Bae IH, et al. A novel polymer-free drug-eluting stent coated with everolimus using nitrogen-doped titanium dioxide film deposition in a porcine coronary restenosis model. Int J Cardiol. 2016; 222:436–440.
Article29. Lee SY, Bae IH, Park DS, Jang EJ, Shim JW, Lim KS, et al. Comparison of dextran-based sirolimus-eluting stents and PLA-based sirolimus-eluting stents in vitro and in vivo. J Biomed Mater Res A. 2017; 105:301–310.
Article30. Lim KS, Park JK, Jeong MH, Hah JW, Kim DG, Bae IH, et al. Comparison of sirolimus loaded PLGA-PEG Co-polymer coronary stent and bare metal stent in a porcine coronary restenosis model. Macromol Res. 2014; 22:639–646.
Article31. Park DS, Park JK, Jeong MH, Bae IH, Lee SY, Jang EJ, et al. Tacrolimus-eluting stent with biodegradable polymer is more effective than sirolimus-and everolimus-eluting stent in rabbit iliac artery restenosis model. Macromol Res. 2015; 23:1034–1041.
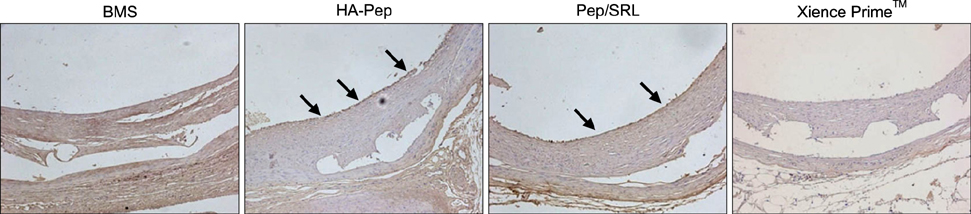
Article32. Jang EJ, Bae IH, Park DS, Lee SY, Lim KS, Park JK, et al. Effect of a novel peptide, WKYMVm- and sirolimus-coated stent on re-endothelialization and anti-restenosis. J Mater Sci Mater Med. 2015; 26:251.
Article33. Sim DS, Kwon JS, Kim YS, Chung HC, Hong YJ, Park HW, et al. Experience with endothelial progenitor cell capturing aptamers for coating of intracoronary stents in a porcine model. Tissue Eng Regen Med. 2009; 6:555–561.34. Kim JM, Bae IH, Lim KS, Park JK, Park DS, Lee SY, et al. A method for coating fucoidan onto bare metal stent and in vivo evaluation. Prog Org Coat. 2015; 78:348–356.
Article35. Bae IH, Park IK, Park DS, Lee H, Jeong MH. Thromboresistant and endothelialization effects of dopamine-mediated heparin coating on a stent material surface. J Mater Sci Mater Med. 2012; 23:1259–1269.
Article36. Kang SN, Kim SE, Choi J, Park K, Goo JH, Sim DS, et al. Comparison of phytoncide with sirolimus as a novel drug candidate for drug-eluting stent. Biomaterials. 2015; 44:1–10.
Article37. Park JK, Lee JH, Nah JW, Kim HK, Lim KS, Bae IH, et al. Development of a novel drug-eluting stent consisting of an abluminal and luminal coating layer dual therapy system. RSC Adv. 2015; 5:40700–40707.
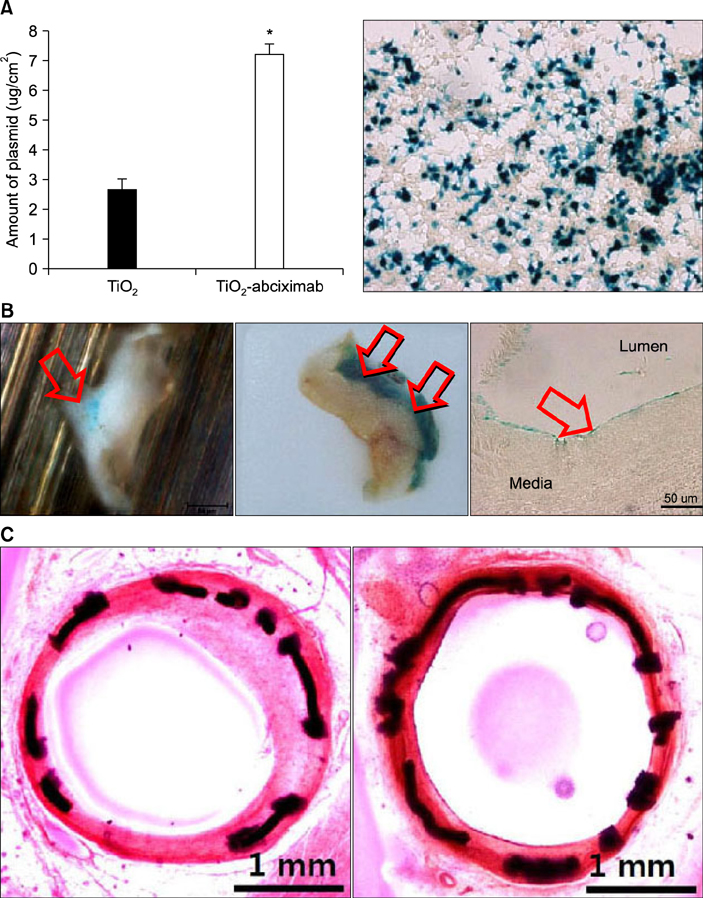
Article38. Kwon JS, Song SJ, Yang EJ, Kim YS, Lim KS, Kim DG, et al. Novel abciximab-Kruppel-like factor 4-plasmid dual-delivery titanium dioxide-coated coronary stent. Int J Cardiol. 2013; 168:5104–5106.
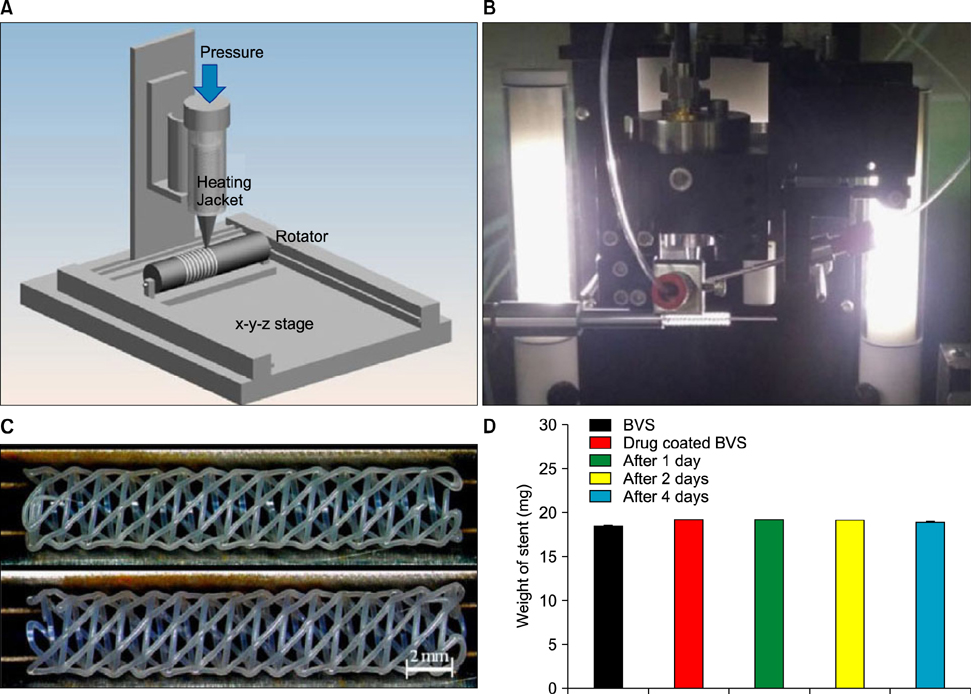
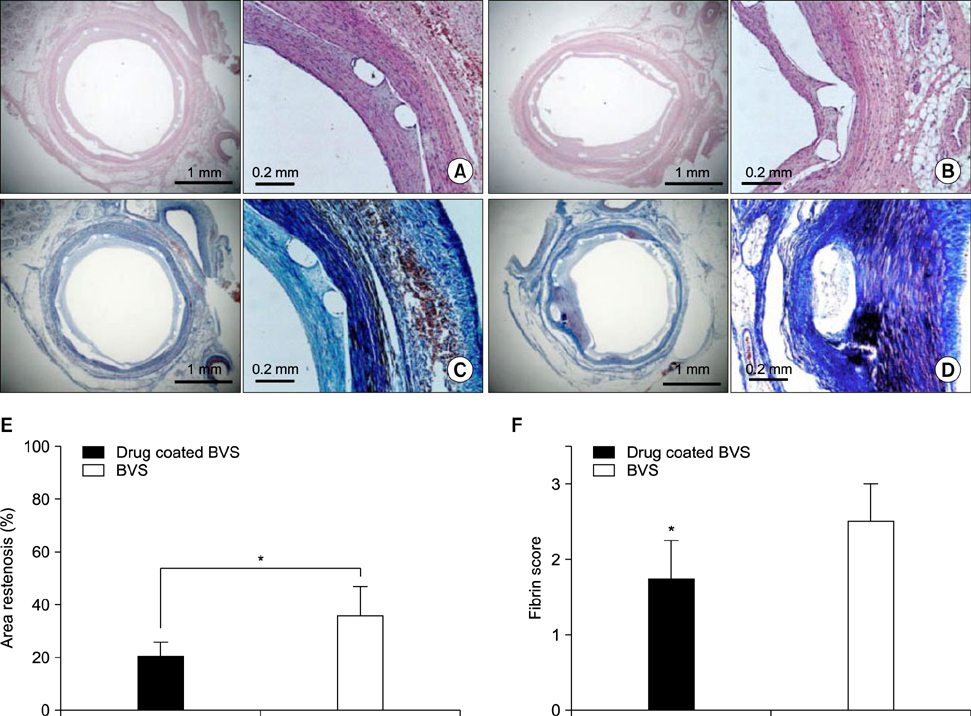
Article39. Che HL, Bae IH, Lim KS, Song IT, Lee H, Muthiah M, et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012; 33:8548–8556.40. Park SA, Lee SJ, Lim KS, Bae IH, Lee JH, Kim WD, et al. In vivo evaluation and characterization of a bio-absorbable drug-coated stent fabricated using a 3D-printing system. Mater Lett. 2015; 141:355–358.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug-Eluting Stent Used to Treat a Case of Recurrent Right Coronary Artery In-Stent Restenoses often Accompanied by Acute Inferior Wall Myocardial Infarction
- A Case of Stent Thrombosis Occurred at 5 Years after Sirolimus-Eluting Stent Implantation
- A Bumpy and Winding but Right Path to Domestic Drug-Eluting Coronary Stents
- Drug-Eluting Stent: Present and Future
- Coronary Stent Thrombosis: Current Insights into New Drug-Eluting Stent Designs