Korean Circ J.
2013 Oct;43(10):645-654. 10.4070/kcj.2013.43.10.645.
A Bumpy and Winding but Right Path to Domestic Drug-Eluting Coronary Stents
- Affiliations
-
- 1Korea Cardiovascular Stent Research Institute of Chonnam National University, Gwangju, Korea. myungho@chollian.net
- KMID: 1826555
- DOI: http://doi.org/10.4070/kcj.2013.43.10.645
Abstract
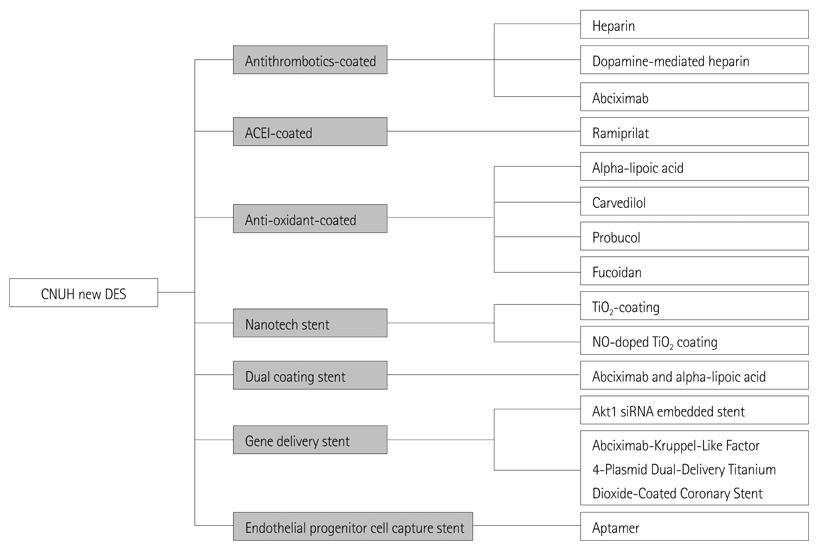
- Restenosis and stent thrombosis remain major concerns after percutaneous coronary intervention for the treatment of coronary artery disease. The present review was undertaken in order to highlight the various coronary stents that have been investigated in our Heart Research Center, and how far we have come from the first heparin-coated stent first used in the late 1990s. Thereafter, from the abciximab-coated stent to the current gene-delivery stent and other newer agents, our group has applied a range of techniques in this field. However, in groups similar to ours, the restenosis rates of such stents are still high for second-generation drug-eluting stents (DESs). Moreover, our nation imports almost all of these types of stents from other countries. Thus, we need to develop domestic coronary stents. Research into newer DESs are warranted in Korea so as to achieve improved safety and efficacy outcomes.
MeSH Terms
Figure
Cited by 2 articles
-
Development of Novel Drug-Eluting Stents for Acute Myocardial Infarction
Doo Sun Sim, Myung Ho Jeong
Chonnam Med J. 2017;53(3):187-195. doi: 10.4068/cmj.2017.53.3.187.Preclinical Evaluation of a Novel Polymer-free Everolimus-eluting Stent in a Mid-term Porcine Coronary Restenosis Model
Kyung Hoon Cho, Myung Ho Jeong, Dae Sung Park, Moonki Kim, JungHa Kim, Jun-Kyu Park, Xiongyi Han, Dae Young Hyun, Min Chul Kim, Doo Sun Sim, Young Joon Hong, Ju Han Kim, Youngkeun Ahn
J Korean Med Sci. 2021;36(40):e259. doi: 10.3346/jkms.2021.36.e259.
Reference
-
1. Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991; 17:758–769.2. Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985; 6:369–375.3. Bae Y, Jeong MH, Jang YS, et al. Comparison of porcine corinary stent restenosis between MAC (Maximum Arterial Re-Creation) and Palmaz-Schatz stent. Korean Circ J. 1998; 28:89–96.4. Giraldo AA, Esposo OM, Meis JM. Intimal hyperplasia as a cause of restenosis after percutaneous transluminal coronary angioplasty. Arch Pathol Lab Med. 1985; 109:173–175.5. Ahn YK, Jeong MH, Kim JW, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999; 48:324–330.6. Hong YJ, Jeong MH, Ahn Y, Kang JC. The efficacy and safety of drug-eluting stents in patients with acute myocardial infarction: results from Korea Acute Myocardial Infarction (KAMIR). Int J Cardiol. 2013; 163:1–4.7. Chen KY, Rha SW, Li YJ, et al. Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction under-going primary percutaneous coronary intervention. Circulation. 2009; 119:3207–3214.8. Park KH, Jeong MH, Lee MG, et al. What is the optimal duration of triple anti-platelet therapy in patients with acute myocardial infarction undergoing drug-eluting stent implantation? J Cardiol. 2011; 57:53–60.9. Lake DF, Briggs AD, Akporiaye ET. Immunopharmacology. In : Katzung BG, editor. Basic & Clinical pharmacology. 12th ed. New York: McGraw Hill;2012. p. 985–994.10. Bonow RO, Mann DL, Zipes DP, Libby P, Braunwald E. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Elsevier Saunders;2012.11. Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002; 41:391–412.12. Vrolix MC, Legrand VM, Reiber JH, et al. MENTOR Trial Investigators. Heparin-coated Wiktor stents in human coronary arteries (MENTOR trial). Am J Cardiol. 2000; 86:385–389.13. Kim YJ, Kang IK, Huh MW, Yoon SC. Surface characterization and in vitro blood compatibility of poly (ethylene terephthalate) immobilized with insulin and/or heparin using plasma glow discharge. Biomaterials. 2000; 21:121–130.14. Chen H, Chen Y, Sheardown H, Brook MA. Immobilization of heparin on a silicone surface through a heterobifunctional PEG spacer. Biomaterials. 2005; 26:7418–7424.15. The EPIC Investigation. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994; 330:956–961.16. The EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N Engl J Med. 1997; 336:1689–1696.17. Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE Study. Lancet. 1997; 349:1429–1435.18. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. N Engl J Med. 1998; 338:1488–1497.19. Tam SH, Sassoli PM, Jordan RE, Nakada MT. Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and alpha(v)beta3 integrins. Circulation. 1998; 98:1085–1091.20. Reverter JC, Béguin S, Kessels H, Kumar R, Hemker HC, Coller BS. Inhibition of platelet-mediated, tissue factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody. Potential implications for the effect of c7E3 Fab treatment on acute thrombosis and "clinical restenosis". J Clin Invest. 1996; 98:863–874.21. Shappell SB, Toman C, Anderson DC, Taylor AA, Entman ML, Smith CW. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990; 144:2702–2711.22. Simon DI, Xu H, Ortlepp S, Rogers C, Rao NK. 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1. Arterioscler Thromb Vasc Biol. 1997; 17:528–535.23. Mickelson JK, Ali MN, Kleiman NS, et al. Chimeric 7E3 Fab (ReoPro) decreases detectable CD11b on neutrophils from patients undergoing coronary angioplasty. J Am Coll Cardiol. 1999; 33:97–106.24. Lefkovits J, Topol EJ. Platelet glycoprotein IIb/IIIa receptor antagonists in coronary artery disease. Eur Heart J. 1996; 17:9–18.25. Pratt RE, Dzau VJ. Pharmacological strategies to prevent restenosis: lessons learned from blockade of the renin-angiotensin system. Circulation. 1996; 93:848–852.26. Nakamura M, Funakoshi T, Arakawa N, Yoshida H, Makita S, Hiramori K. Effect of angiotensin-converting enzyme inhibitors on endothelium-dependent peripheral vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 1994; 24:1321–1327.27. Hirooka Y, Imaizumi T, Masaki H, et al. Captopril improves impaired endothelium-dependent vasodilation in hypertensive patients. Hypertension. 1992; 20:175–180.28. Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996; 94:258–265.29. Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv Pharmacol. 1997; 38:79–101.30. Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999; 22:1296–1301.31. Sung MJ, Kim W, Ahn SY, et al. Protective effect of alpha-lipoic acid in lipopolysaccharide-induced endothelial fractalkine expression. Circ Res. 2005; 97:880–890.32. Lee KM, Park KG, Kim YD, et al. Alpha-lipoic acid inhibits fractalkine expression and prevents neointimal hyperplasia after balloon injury in rat carotid artery. Atherosclerosis. 2006; 189:106–114.33. Ohlstein EH, Douglas SA, Sung CP, et al. Carvedilol, a cardiovascular drug, prevents vascular smooth muscle cell proliferation, migration, and neointimal formation following vascular injury. Proc Natl Acad Sci U S A. 1993; 90:6189–6193.34. Sung CP, Arleth AJ, Ohlstein EH. Carvedilol inhibits vascular smooth muscle cell proliferation. J Cardiovasc Pharmacol. 1993; 21:221–227.35. Sung CP, Arleth AJ, Eichman C, Truneh A, Ohlstein EH. Carvedilol, a multiple-action neurohumoral antagonist, inhibits mitogen-activated protein kinase and cell cycle progression in vascular smooth muscle cells. J Pharmacol Exp Ther. 1997; 283:910–917.36. Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet. 2003; 361:247–249.37. Pfuetze KD, Dujovne CA. Probucol. Curr Atheroscler Rep. 2000; 2:47–57.38. Tardif JC, Cöté G, Lespérance J, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 1997; 337:365–372.39. Watanabe K, Sekiya M, Ikeda S, Miyagawa M, Hashida K. Preventive effects of probucol on restenosis after percutaneous transluminal coronary angioplasty. Am Heart J. 1996; 132(1 Pt 1):23–29.40. Côté G, Tardif JC, Lespérance J, et al. Effects of probucol on vascular remodeling after coronary angioplasty. Multivitamins and Protocol Study Group. Circulation. 1999; 99:30–35.41. Schneider JE, Berk BC, Gravanis MB, et al. Probucol decreases neointimal formation in a swine model of coronary artery balloon injury. A possible role for antioxidants in restenosis. Circulation. 1993; 88:628–637.42. Wasserman MA, Sundell CL, Kunsch C, Edwards D, Meng CQ, Medford RM. Chemistry and pharmacology of vascular protectants: a novel approach to the treatment of atherosclerosis and coronary artery disease. Am J Cardiol. 2003; 91:34A–40A.43. Tardif JC, Grégoire J, Schwartz L, et al. Effects of AGI-1067 and probucol after percutaneous coronary interventions. Circulation. 2003; 107:552–558.44. Wakeyama T, Ogawa H, Iida H, et al. Effects of candesartan and probucol on restenosis after coronary stenting: results of insight of stent intimal hyperplasia inhibition by new angiotensin II receptor antagonist (ISHIN) trial. Circ J. 2003; 67:519–524.45. Logeart D, Prigent-Richard S, Jozefonvicz J, Letourneur D. Fucans, sulfated polysaccharides extracted from brown seaweeds, inhibit vascular smooth muscle cell proliferation. I. Comparison with heparin for antiproliferative activity, binding and internalization. Eur J Cell Biol. 1997; 74:376–384.46. Kim JM, Cho EJ, et al. Effect of fucoidan for smooth muscle cell proliferation and neointima hyperplasia in a rabbit iliac artery in-stent restenosis model. Korean Circ J. 2011; 41:II-245. Abstract.47. Liu Q, Ding J, Mante FK, Wunder SL, Baran GR. The role of surface functional groups in calcium phosphate nucleation on titanium foil: a self-assembled monolayer technique. Biomaterials. 2002; 23:3103–3111.48. Fu J, Ji J, Yuan W, Shen J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials. 2005; 26:6684–6692.49. Meng S, Liu Z, Shen L, et al. The effect of a layer-by-layer chitosan-heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials. 2009; 30:2276–2283.50. Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007; 318:426–430.51. Rich DH, Singh J. The Carbodiimide Method. In : Meienhofer , editor. The peptides. Acadenic Press;1979. p. 241–261.52. Bae IH, Park IK, Park DS, Lee H, Jeong MH. Thromboresistant and endothelialization effects of dopamine-mediated heparin coating on a stent material surface. J Mater Sci Mater Med. 2012; 23:1259–1269.53. Song SJ, Park YJ, Park J, et al. Preparation of a drug-eluting stent using a TiO2 film deposited by plasma enhanced chemical vapour deposition as a drug-combining matrix. J Mater Chem. 2010; 20:4792–4801.54. Windecker S, Mayer I, De Pasquale G, et al. Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation. 2001; 104:928–933.55. Tsyganov I, Maitz MF, Wieser E, Richter E, Reuther H. Correlation between blood compatibility and physical surface properties of titanium-based coatings. Surf Coat Technol. 2005; 200:1041–1044.56. Puskas JE, Muñoz-Robledo LG, Hoerr RA, et al. Drug-eluting stent coatings. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009; 1:451–462.57. Song SJ, Jung KW, Park YJ, et al. Nitrogen-doped TiO2 films as drug-binding matrices for the preparation of drug-eluting stents. J Mater Chem. 2011; 21:8169–8177.58. Packer L, Witt EH, Tritschler HJ. Alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995; 19:227–250.59. Sim DS, Kwon JS, Kim YS, et al. Experience with Endothelial Progenitor Cell Capturing Aptamers for Coating of Intracoronary Stents in a Porcine Model. Tissue Eng Regen Med. 2009; 6:555–561.60. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007; 370:937–948.61. Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimusand paclitaxel-eluting coronary stents. N Engl J Med. 2007; 356:998–1008.62. Räber L, Magro M, Stefanini GG, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012; 125:1110–1121.63. Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010; 375:201–209.64. Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010; 362:1663–1674.65. Jensen LO, Thayssen P, Hansen HS, et al. Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). Circulation. 2012; 125:1246–1255.66. Kimura T, Morimoto T, Natsuaki M, et al. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-eluting Versus Everolimus-eluting stent Trial (RESET). Circulation. 2012; 126:1225–1236.67. Leon MB, Nikolsky E, Cutlip DE, et al. Improved late clinical safety with zotarolimus-eluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-up from the ENDEAVOR IV (Randomized Comparison of Zotarolimus- and Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2010; 3:1043–1050.68. Park DW, Kim YH, Yun SC, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. J Am Coll Cardiol. 2010; 56:1187–1195.69. Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012; 125:2873–2891.70. Camenzind E, Wijns W, Mauri L, et al. Stent thrombosis and major clinical events at 3 years after zotarolimus-eluting or sirolimus-eluting coronary stent implantation: a randomised, multicentre, open-label, controlled trial. Lancet. 2012; 380:1396–1405.71. Lim SY, Bae EH, Jeong MH, et al. Effect of alpha lipoic acid in a porcine in-stent restenosis model. J Cardiol. 2009; 54:375–385.72. Kim W, Jeong MH, Hong YJ, et al. The long-term clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (Abciximab: Reopro) coated stent in patients with coronary artery disease. Korean J Intern Med. 2004; 19:220–229.73. Kim W, Jeong MH, Kim KH, et al. The clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (abciximab: ReoPro)-coated stent in acute myocardial infarction. J Am Coll Cardiol. 2006; 47:933–938.74. Hong YJ, Jeong MH, Hwang SH, et al. Impact of postprocedure minimum stent area on long-term results following abciximab-coated stent implantation: an intravascular ultrasound analysis. Int J Cardiol. 2007; 123:23–28.75. Hong YJ, Jeong MH, Lee SR, et al. Anti-inflammatory effect of abciximab-coated stent in a porcine coronary restenosis model. J Korean Med Sci. 2007; 22:802–809.76. Kim SS, Hong YJ, Jeong MH, et al. Two-year clinical outcome after abciximab-coated stent implantation in patients with coronary artery disease. Circ J. 2010; 74:442–448.77. Hong YJ, Jeong MH, Song SJ, et al. Effects of ramiprilat-coated stents on neointimal hyperplasia, inflammation, and arterial healing in a porcine coronary restenosis model. Korean Circ J. 2011; 41:535–541.78. Kim JM, Kim JH, Jeong MH, et al. Fabrication and evaluation of a fucoidan-coated stent in a porcine coronary restenosis model. Korean Circ J. 2012; 42:II-120. Abstract.79. Lim KS, Hong YJ, Hachinohe D, et al. Effect of a dual drug-coated stent with abciximab and alpha-lipoic Acid in a porcine coronary restenosis model. Korean Circ J. 2011; 41:241–247.80. Räber L, Kelbæk H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012; 308:777–787.81. Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013; 381:651–660.82. Stefanini GG, Kalesan B, Serruys PW, et al. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trial. Lancet. 2011; 378:1940–1948.83. Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012; 33:1214–1222.84. Che HL, Bae IH, Lim KS, et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012; 33:8548–8556.85. Jiang HL, Hong SH, Kim YK, et al. Aerosol delivery of spermine-based poly(amino ester)/Akt1 shRNA complexes for lung cancer gene therapy. Int J Pharm. 2011; 420:256–265.86. Jere D, Arote R, Jiang HL, Kim YK, Cho MH, Cho CS. Biodegradable nano-polymeric system for efficient Akt1 siRNA delivery. J Nanosci Nanotechnol. 2010; 10:3366–3369.87. Jiang HL, Xu CX, Kim YK, et al. The suppression of lung tumorigenesis by aerosol-delivered folate-chitosan-graft-polyethylenimine/Akt1 shRNA complexes through the Akt signaling pathway. Biomaterials. 2009; 30:5844–5852.88. Duguay D, deBlois D. Differential regulation of Akt, caspases and MAP kinases underlies smooth muscle cell apoptosis during aortic remodelling in SHR treated with amlodipine. Br J Pharmacol. 2007; 151:1315–1323.89. Jere D, Jiang HL, Kim YK, et al. Chitosan-graft-polyethylenimine for Akt1 siRNA delivery to lung cancer cells. Int J Pharm. 2009; 378:194–200.90. Kwon JC, Song SJ, Yang EJ, et al. Novel abciximab-Kruppel-like factor 4-plasmid dual-delivery titanium dioxide-coated coronary stent. Int J Cardiol. 2013; [Epub ahead of print].
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis
- Is There a Benefit of Drug-Eluting Stents Rather than Bare Metal Stents in Large Coronary Artery Lesions?
- Angiographic spontaneous pseudo-resolution of a coronary artery aneurysm after implantation of a sirolimus-eluting stent
- Treatment of Diffuse Long Coronary Artery Disease with Overlapping Drug-Eluting Stents is Effective?
- Novel Coronary Stent Platforms