J Korean Ophthalmol Soc.
2017 Sep;58(9):1036-1041. 10.3341/jkos.2017.58.9.1036.
Change of Outer Retinal Thickness in Fellow Eyes of Patients with Unilateral Age-related Macular Degeneration
- Affiliations
-
- 1Siloam Eye Hospital, Seoul, Korea. hjpeye@siloam.co.kr
- 2Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2390066
- DOI: http://doi.org/10.3341/jkos.2017.58.9.1036
Abstract
- PURPOSE
To compare the outer retinal thickness in normal fellow eyes of patients with unilateral age-related macular degeneration (AMD) and normal control eyes.
METHODS
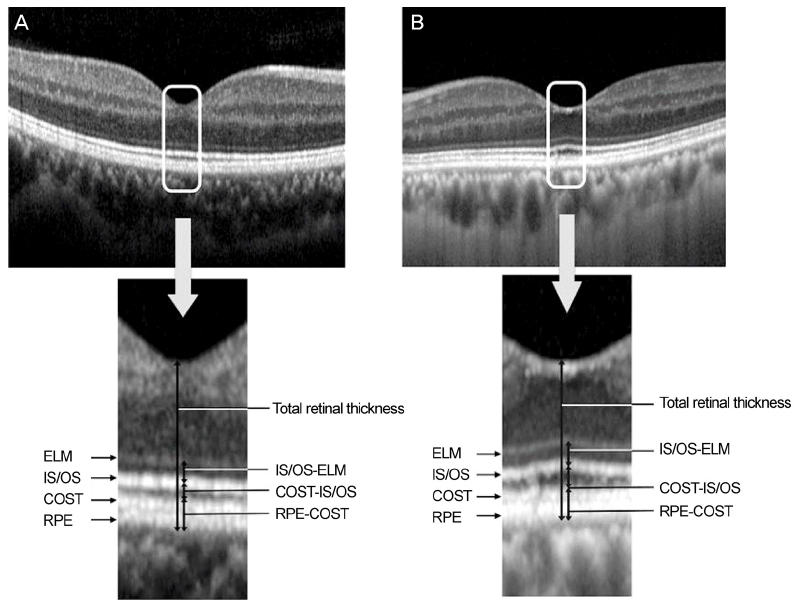
We retrospectively reviewed the medical records of 60 patients with unilateral exudative AMD including polypoidal choroidal vasculopathy and 60 normal controls. Spectralis optical coherence tomography was performed in the normal fellow eyes of patients with unilateral AMD and in the normal group. The thicknesses between the retinal pigment epithelium (RPE) line and the cone outer segment tips (COST) line, between the COST line and the photoreceptor inner segment/outer segment (IS/OS) line, and between the IS/OS line and the external limiting membrane (ELM) line were measured at the fovea in both groups.
RESULTS
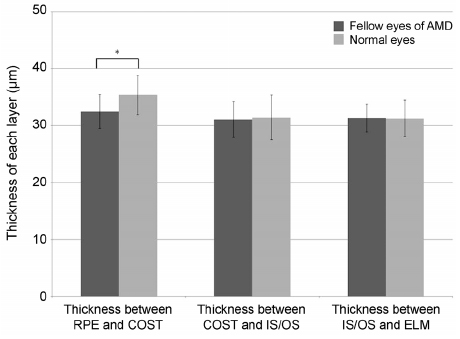
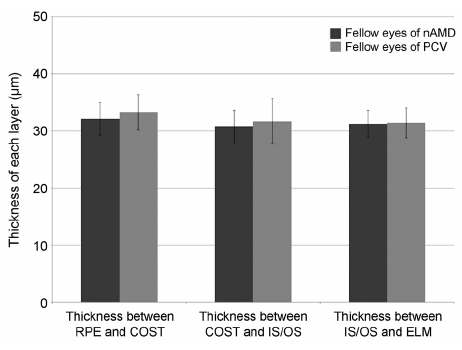
The thickness between the RPE and COST lines was 32.4 ± 3.0 µm in normal fellow eyes of patients with unilateral AMD and 35.3 ± 3.5 µm in the normal group (p < 0.001). Total retinal thickness, thicknesses between the COST and the IS/OS lines and the IS/OS and the ELM lines in fellow eyes were not significantly different from those of normal eyes (p = 0.126, 0.615, 0.874). There was no significant difference in total retinal thickness or each outer retinal thickness measured in normal fellow eyes between patients with neovascular AMD and polypoidal choroidal vasculopathy.
CONCLUSIONS
The thickness between the RPE and the COST lines was thinner in the fellow eyes of patients with unilateral AMD than in the normal eyes. We suggest that less thickness between the RPE and COST lines might indicate a greater risk of AMD.
Keyword
MeSH Terms
Figure
Reference
-
1. Submacular Surgery, Solomon SD, Jefferys JL, et al. Incident choroidal neovascularization in fellow eyes of patients with unilateral subfoveal choroidal neovascularization secondary to age-related macular degeneration: SST report No. 20 from the Submacular Surgery Trials Research Group. Arch Ophthalmol. 2007; 125:1323–1330.2. Macular Photocoagulation Study Group. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Arch Ophthalmol. 1993; 111:1189–1199.3. Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997; 115:741–747.4. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997; 104:7–21.5. Klein R, Knudtson MD, Klein BE. Statin use and the five-year incidence and progression of age-related macular degeneration. Am J Ophthalmol. 2007; 144:1–6.6. Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology. 2004; 111:1176–1182.7. van Leeuwen R, Klaver CC, Vingerling JR, et al. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol. 2003; 121:519–526.8. Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002; 120:1435–1442.9. Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20:705–732.10. Acton JH, Smith RT, Hood DC, Greenstein VC. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53:7618–7624.11. Kenmochi J, Ito Y, Terasaki H. Changes of outer retinal thickness with increasing age in normal eyes and in normal fellow eyes of patients with unilateral age-related macular degeneration. Retina. 2017; 37:47–52.12. Ramrattan RS, van der Schaft TL, Mooy CM, et al. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994; 35:2857–2864.13. Friedman E, Ts'o MO. The retinal pigment epithelium. II. Histologic changes associated with age. Arch Ophthalmol. 1968; 79:315–320.14. Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000; 41:2015–2018.15. Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976; 60:324–341.16. Pinilla I, Garcia-Martin E, Fernandez-Larripa S, et al. Reproducibility and repeatability of Cirrus and Spectralis Fourier-domain optical coherence tomography of healthy and epiretinal membrane eyes. Retina. 2013; 33:1448–1455.17. Hafner J, Prager S, Lammer J, et al. Comparison of ganglion cell inner plexiform layer thickness by Cirrus and Spectralis optical coherence tomography in diabetic macular edema. Retina. 2017; 04. 03. DOI: 10.1097/IAE.0000000000001631. [Epub ahead of print].18. Barbazetto IA, Saroj N, Shapiro H, et al. Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. Am J Ophthalmol. 2010; 149:939–946.e1.19. Lee JJ, Lew YJ, Cho SW, Kim JW. Incidence of new choroidal neovascularization in fellow eyes of patients treated for age-related macular degeneration. J Korean Ophthalmol Soc. 2013; 54:1534–1539.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Clinical Study on the Fellow Eyes in Unilateral Retinal Detahment
- Lamina Cribrosa Thickness in the Fellow Eyes of Patients with Unilateral Retinal Vein Occlusion
- Incidence of New Choroidal Neovascularization in Fellow Eyes of Patients Treated for Age-Related Macular Degeneration
- The Analysis of Peripapillary RNFL, Macula and Macular Ganglion Cell Layer Thickness in Patients with Monocular Amblyopia Using SD-OCT
- Analysis of Retinal Capillary Using Optical Coherence Tomographic Angiography of Unilateral Normal Tension Glaucoma