Investig Clin Urol.
2017 May;58(3):164-170. 10.4111/icu.2017.58.3.164.
Updated clinical results of active surveillance of very-low-risk prostate cancer in Korean men: 8 years of follow-up
- Affiliations
-
- 1Department of Urology, Keimyung University School of Medicine, Daegu, Korea. cikim@dsmc.or.kr
- KMID: 2388060
- DOI: http://doi.org/10.4111/icu.2017.58.3.164
Abstract
- PURPOSE
Update and reanalysis of our experience of active surveillance (AS) for prostate cancer (PCa) in Korea.
MATERIALS AND METHODS
A prospective, single-arm, cohort study was initiated in January 2008. Patients were selected according to the following criteria: Gleason sum ≤6 with single positive core with ≤30% core involvement, clinical stage≤T1c, prostate-specific antigen (PSA)≤10 ng/mL, and negative magnetic resonance imaging (MRI) results. Follow-up was by PSA measurement every 6 months, prostate biopsies at 1 year and then every 2-3 years, and MRI every year.
RESULTS
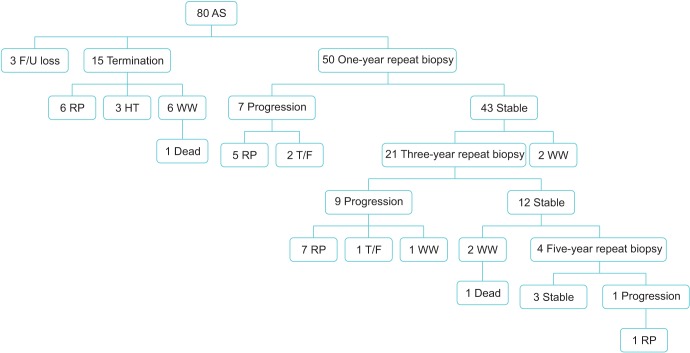
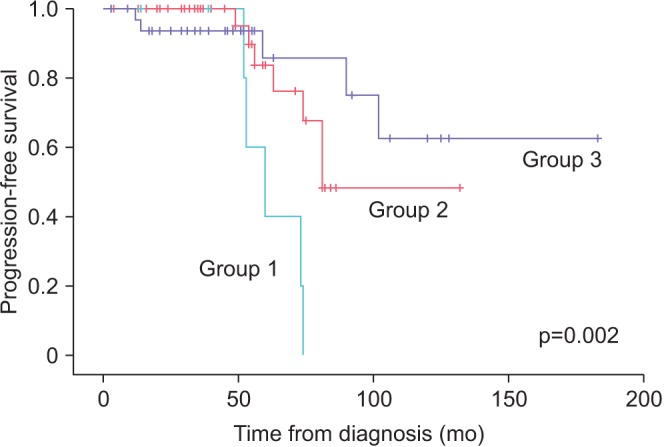
A total of 80 patients were treated with AS. Median follow-up was 52 months (range, 6-96 months). Of them, 39 patients (48.8%) discontinued AS for various reasons (17, disease progression; 9, patient preference; 10, watchful waiting due to old age; 3, follow-up loss; 2, death). The probability of progression was 14.0% and 42.9% at 1 and 3 years, respectively. Overall survival was 97.5%. PCa-specific survival was 100%. Progression occurred in 5 of 7 patients (71.4%) with a prostate volume less than 30 mL, 7 of 40 patients (17.5%) with a prostate volume of 30 to 50 mL, and 5 of 33 patients (15.2%) with a prostate volume of 50 mL or larger. There were 8 detectable positive lesions on follow-up MRI. Of them, 6 patients (75%) had actual progressed disease.
CONCLUSIONS
Small prostate volume was associated with a tendency for cancer progression. MRI was helpful and promising for managing AS. Nevertheless, regular biopsies should be performed. AS is a safe and feasible treatment option for very-low-risk PCa in Korea. However, AS should continue to be used in carefully selected patients.
MeSH Terms
Figure
Reference
-
1. Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012; 366:981–990. PMID: 22417251.
Article2. Roobol MJ, Kerkhof M, Schröder FH, Cuzick J, Sasieni P, Hakama M, et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). Eur Urol. 2009; 56:584–591. PMID: 19660851.
Article3. Klotz L. Active surveillance versus radical treatment for favorable-risk localized prostate cancer. Curr Treat Options Oncol. 2006; 7:355–362. PMID: 16904052.
Article4. Lawrentschuk N, Klotz L. Active surveillance for low-risk prostate cancer: an update. Nat Rev Urol. 2011; 8:312–320. PMID: 21519351.
Article5. Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 157:120–134. PMID: 22801674.
Article6. Chung JS, Choi HY, Song HR, Byun SS, Seo Si, Song C, et al. Preoperative nomograms for predicting extracapsular extension in Korean men with localized prostate cancer: a multi-institutional clinicopathologic study. J Korean Med Sci. 2010; 25:1443–1448. PMID: 20890424.
Article7. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010; 28:126–131. PMID: 19917860.
Article8. Loeb S, Bruinsma SM, Nicholson J, Briganti A, Pickles T, Kakehi Y, et al. Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol. 2015; 67:619–626. PMID: 25457014.
Article9. Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013; 31:2991–2997. PMID: 23775960.
Article10. Hwang I, Lim D, Jeong YB, Park SC, Noh JH, Kwon DD, et al. Upgrading and upstaging of low-risk prostate cancer among Korean patients: a multicenter study. Asian J Androl. 2015; 17:811–814. PMID: 25578934.11. Ahn HJ, Ko YH, Jang HA, Kang SG, Kang SH, Park HS, et al. Single positive core prostate cancer in a 12-core transrectal biopsy scheme: clinicopathological implications compared with multifocal counterpart. Korean J Urol. 2010; 51:671–676. PMID: 21031085.
Article12. Lee SE, Kim DS, Lee WK, Park HZ, Lee CJ, Doo SH, et al. Application of the Epstein criteria for prediction of clinically insignificant prostate cancer in Korean men. BJU Int. 2010; 105:1526–1530. PMID: 19912202.
Article13. Ha JY, Kim BH, Park CH, Kim CI. Early experience with active surveillance in low-risk prostate cancer treated. Korean J Urol. 2014; 55:167–171. PMID: 24648870.
Article14. Schillaci O, Calabria F, Tavolozza M, Caracciolo CR, Finazzi Agrò E, Miano R, et al. Influence of PSA, PSA velocity and PSA doubling time on contrast-enhanced 18F-choline PET/CT detection rate in patients with rising PSA after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2012; 39:589–596. PMID: 22231016.
Article15. Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012; 367:203–213. PMID: 22808955.
Article16. Dall'Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012; 62:976–983. PMID: 22698574.17. Kang M, Song B, Lee I, Lee SE, Byun SS, Hong SK. Predictors of pathological upgrading in low-risk prostate cancer patients without hypointense lesions on an apparent diffusion coefficient map of multiparametric magnetic resonance imaging. World J Urol. 2016; 34:1541–1546. PMID: 27074937.
Article18. Babaian KN. Active surveillance for prostate cancer: when to recommend delayed intervention. Asian J Androl. 2015; 17:885–887. PMID: 26178391.
Article19. Ha YS, Yu J, Salmasi AH, Patel N, Parihar J, Singer EA, et al. Prostate-specific antigen density toward a better cutoff to identify better candidates for active surveillance. Urology. 2014; 84:365–371. PMID: 24925834.
Article20. Tosoian JJ, Sundi D, Trock BJ, Landis P, Epstein JI, Schaeffer EM, et al. Pathologic outcomes in favorable-risk prostate cancer: comparative analysis of men electing active surveillance and immediate surgery. Eur Urol. 2016; 69:576–581. PMID: 26456680.
Article21. Jin BS, Kang SH, Kim DY, Oh HG, Kim CI, Moon GH, et al. Pathological upgrading in prostate cancer patients eligible for active surveillance: does prostate-specific antigen density matter? Korean J Urol. 2015; 56:624–629. PMID: 26366274.
Article22. Bjurlin MA, Mendhiratta N, Wysock JS, Taneja SS. Multiparametric MRI and targeted prostate biopsy: improvements in cancer detection, localization, and risk assessment. Cent European J Urol. 2016; 69:9–18.
Article23. Jeong CW, Park YH, Hwang SI, Lee S, Jeong SJ, Hong SK, et al. The role of 3-tesla diffusion-weighted magnetic resonance imaging in selecting prostate cancer patients for active surveillance. Prostate Int. 2014; 2:169–175. PMID: 25599072.
Article24. Henderson DR, de Souza NM, Thomas K, Riches SF, Morgan VA, Sohaib SA, et al. Nine-year follow-up for a study of diffusion-weighted magnetic resonance imaging in a prospective prostate cancer active surveillance cohort. Eur Urol. 2016; 69:1028–1033. PMID: 26482887.
Article25. Marliere F, Puech P, Benkirane A, Villers A, Lemaitre L, Leroy X, et al. The role of MRI-targeted and confirmatory biopsies for cancer upstaging at selection in patients considered for active surveillance for clinically low-risk prostate cancer. World J Urol. 2014; 32:951–958. PMID: 24817183.
Article26. Kim M, Choi SK, Park M, Shim M, Song C, Jeong IG, et al. Characteristics of anteriorly located prostate cancer and the usefulness of multiparametric magnetic resonance imaging for diagnosis. J Urol. 2016; 196:367–373. PMID: 26997311.
Article27. Quon JS, Moosavi B, Khanna M, Flood TA, Lim CS, Schieda N. False positive and false negative diagnoses of prostate cancer at multi-parametric prostate MRI in active surveillance. Insights Imaging. 2015; 6:449–463.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Active Surveillance for Favorable-Risk Prostate Cancer: A Short Review
- Multiparametric MRI in Active Surveillance of Prostate Cancer: An Overview and a Practical Approach
- Outcomes of Active Surveillance in Localized Prostate Cancer
- Clinical Utility of Novel Biomarkers in the Era of Active Surveillance of Prostate Cancer
- Impact of family history of prostate cancer on disease progression for prostatic cancer patients undergoing active surveillance: A systematic review and meta-analysis