J Korean Ophthalmol Soc.
2017 Jul;58(7):836-845. 10.3341/jkos.2017.58.7.836.
Diagnostic Accuracies of Bruch Membrane Opening-minimum Rim Width and Retinal Nerve Fiber Layer Thickness in Glaucoma
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine, Busan, Korea. glaucoma@pnu.ac.kr
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 2387158
- DOI: http://doi.org/10.3341/jkos.2017.58.7.836
Abstract
- PURPOSE
To compare the diagnostic capability of Bruch membrane opening-minimum rim width (BMO-MRW) and peripapillary retinal nerve fiber layer (RNFL) thickness for the detection of primary open angle glaucoma.
METHODS
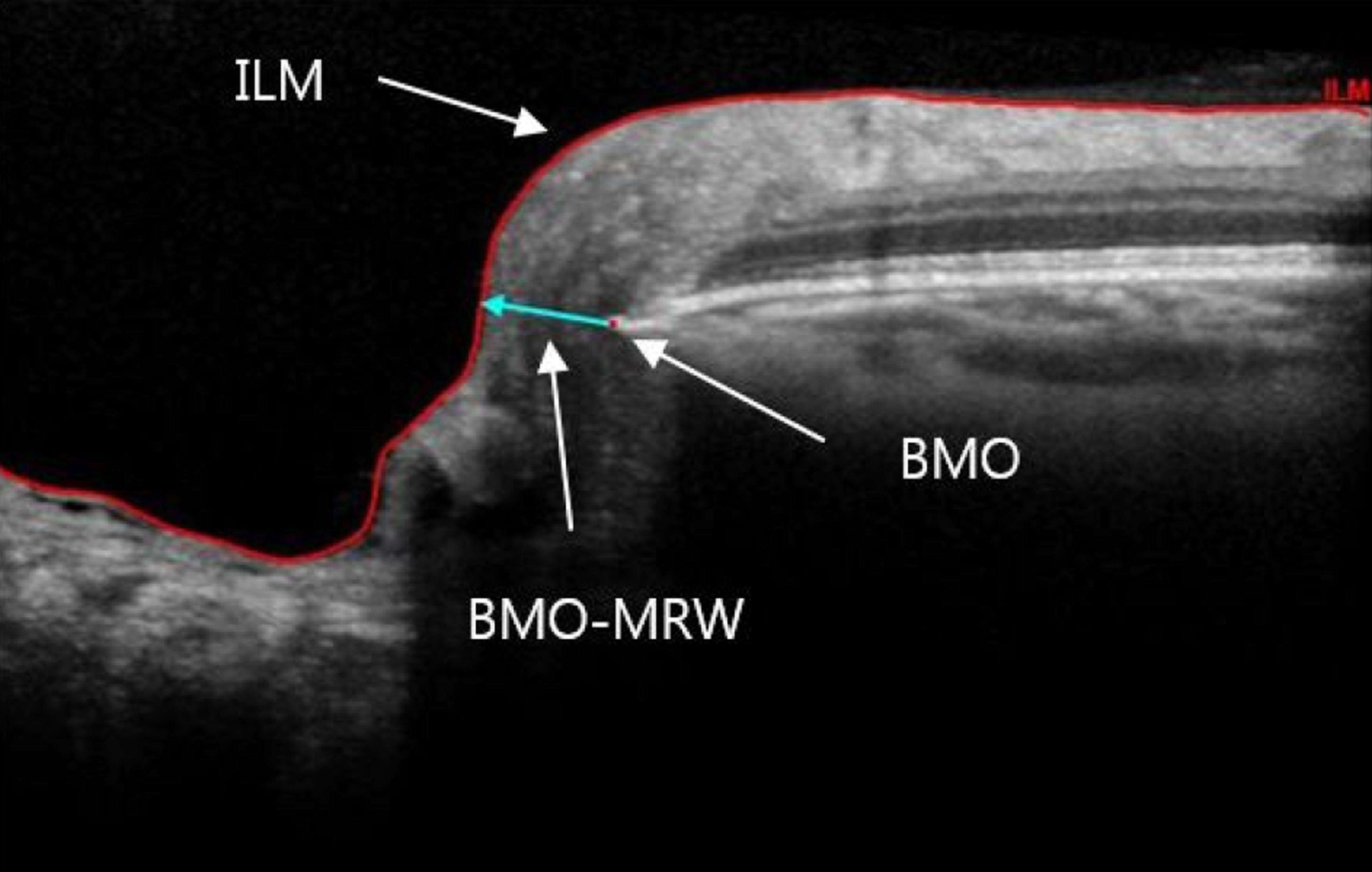
Spectral-domain optical coherence tomography (SD-OCT) with 24 radial and 1 peripapillary B-scans centered on the Bruch membrane opening (BMO) was performed. Two SD-OCT parameters were computed globally and sectorally: (1) BMO-MRW, the minimum distance between BMO and internal limiting membrane; and (2) peripapillary retinal nerve fiber layer (RNFL) thickness. The diagnostic performance of BMO-MRW and RNFL thickness were compared with receiver operating characteristic (ROC) analysis globally and sectorally. Areas under the ROC (AUC) were calculated and compared.
RESULTS
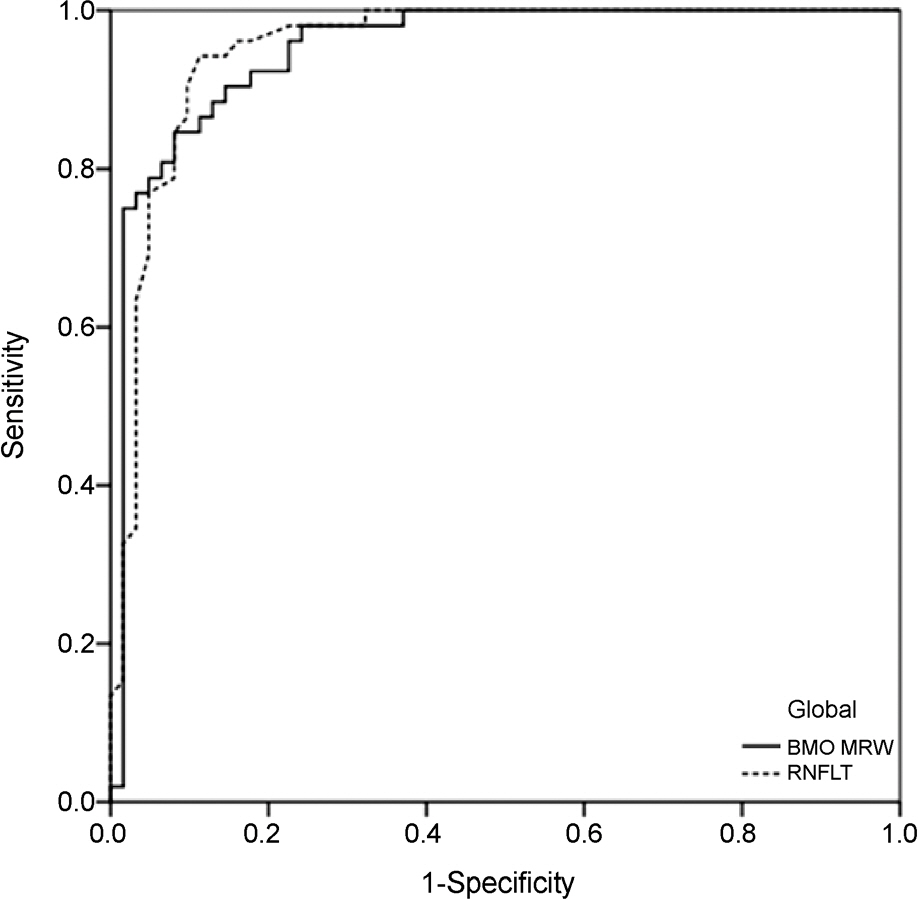
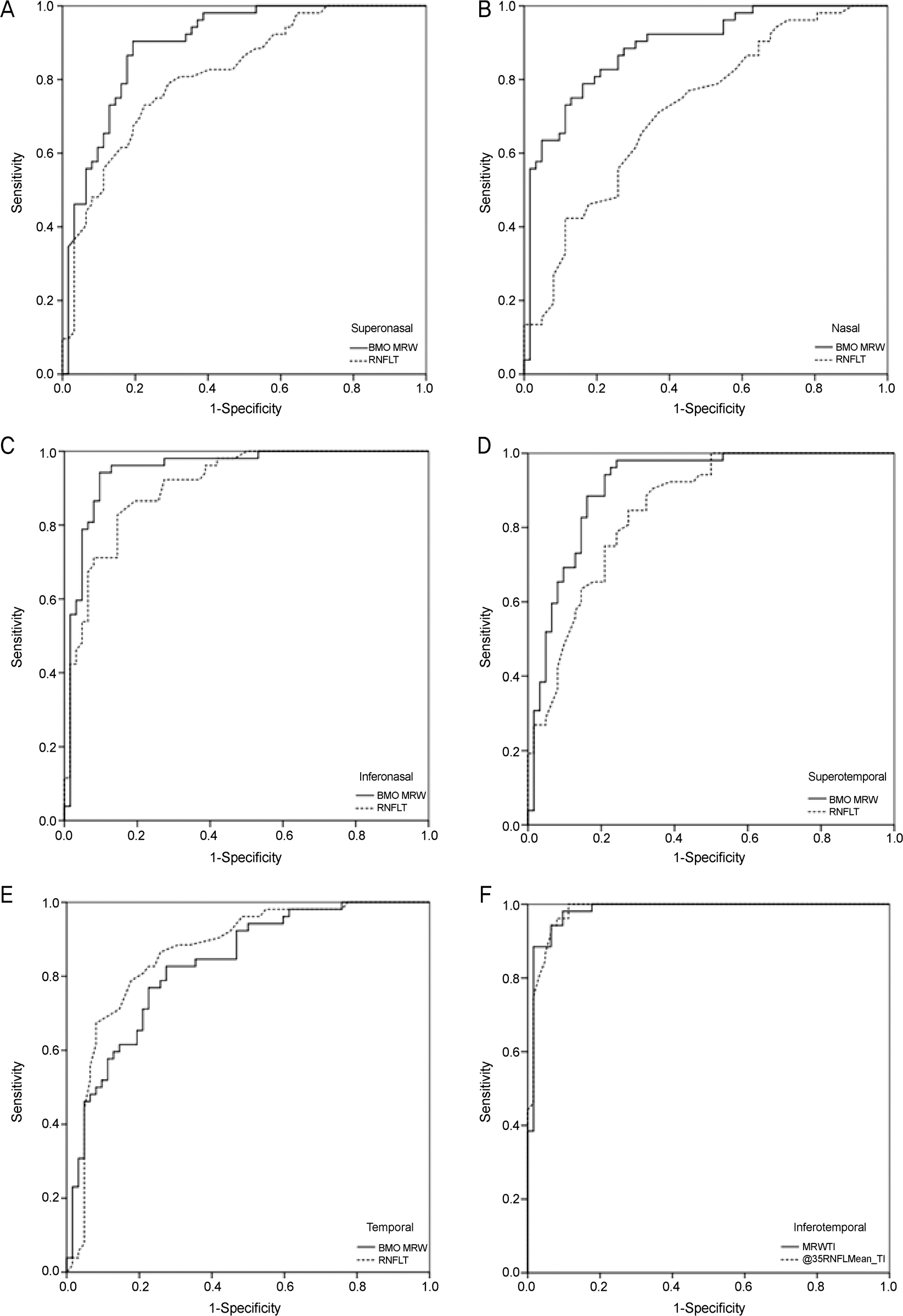
One hundred fourteen eyes (52 healthy, 62 glaucomatous) of 114 participants were included. In global analyses, the performance of BMO-MRW was similar to that of RNFL thickness (AUC 0.95 [95% confidence interval {CI}, 0.91-0.99], and 0.95 [95% CI, 0.91-0.99], respectively, p=0.93). In sectoral analyses, the pair-wise comparison among the ROC curves showed no statistical difference for all sectors except for the superotemporal, superonasal, and nasal sectors, which had significantly larger AUCs in BMO-MRW compared to RNFL thickness (p=0.03, p<0.001, and p=0.03, respectively). The parameter with the largest AUC was the inferotemporal sector for both BMO-MRW and RNFL thickness (AUC 0.98 [95% CI, 0.96-1.00], and 0.98 [95% CI, 0.96-1.00], respectively, p=0.99).
CONCLUSIONS
Global BMO-MRW performed as well as global RNFL thickness for detection of glaucoma. In superotemporal, superonasal and nasal sectors, regional BMO-MRW performed better than regional RNFL thickness.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Sommer A, Miller NR, Pollack I. . The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol. 1977; 95:2149–56.
Article2. Quigley HA, Addicks EM, Green WR. Optic nerve damage in hu-man glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982; 100:135–46.
Article3. Chauhan BC, O'Leary N, Almobarak FA. . Enhanced detection of open-angle glaucoma with an anatomically accurate optical coher-ence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013; 120:535–43.
Article4. Reis AS, Sharpe GP, Yang H. . Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology. 2012; 119:738–47.
Article5. Mizumoto K, Gosho M, Zako M. Correlation between optic nerve head structural parameters and glaucomatous visual field indices. Clin Ophthalmol. 2014; 8:1203–8.6. Pollet-Villard F, Chiquet C, Romanet JP. . Structure-function relationships with spectral-domain optical coherence tomography retinal nerve fiber layer and optic nerve head measurements. Invest Ophthalmol Vis Sci. 2014; 55:2953–62.
Article7. Gardiner SK, Ren R, Yang H. . A method to estimate the amount of neuroretinal rim tissue in glaucoma: comparison with current methods for measuring rim area. Am J Ophthalmol. 2014; 157:540–9.e1-2..
Article8. Danthurebandara VM, Sharpe GP, Hutchison DM. . Enhanced structure-function relationship in glaucoma with an anatomically and geometrically accurate neuroretinal rim measurement. Invest Ophthalmol Vis Sci. 2014; 56:98–105.
Article9. Kim M, Kim TW, Weinreb RN, Lee EJ. Differentiation of para-papillary atrophy using spectral-domain optical coherence tomography. Ophthalmology. 2013; 120:1790–7.
Article10. Knighton RW, Qian C. An optical model of the human retinal nerve fiber layer: implications of directional reflectance for variability of clinical measurements. J Glaucoma. 2000; 9:56–62.
Article11. Malik R, Swanson WH, Garway‐ Heath DF. ‘Structure-function re-lationship’ in glaucoma: past thinking and current concepts. Clin Exp Ophthalmol. 2012; 40:369–80.
Article12. Jeoung JW, Kim TW, Kang KB. . Overlapping of retinal nerve fibers in the horizontal plane. Invest Ophthalmol Vis Sci. 2008; 49:1753–7.
Article13. He L, Ren R, Yang H. . Anatomic vs. acquired image frame dis-cordance in spectral domain optical coherence tomography mini-mum rim measurements. PLoS One. 2014; 9:e92225.14. Chauhan BC, Burgoyne CF. From clinical examination of the optic disc to clinical assessment of the optic nerve head: a paradigm change. Am J Ophthalmol. 2013; 156:218–27.e2..
Article15. Malik R, Belliveau AC, Sharpe GP. . Diagnostic accuracy of optical coherence tomography and scanning laser tomography for identifying glaucoma in myopic eyes. Ophthalmology. 2016; 123:1181–9.
Article16. Yarmohammadi A, Zangwill LM, Diniz-Filho A. . Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology. 2017; 124:709–19.
Article17. Kook MS, Sung K, Kim S. . Study of retinal nerve fibre layer thickness in eyes with high tension glaucoma and hemifield defect. Br J Ophthalmol. 2001; 85:1167–70.
Article18. Takagi ST, Kita Y, Yagi F, Tomita G. Macular retinal ganglion cell complex damage in the apparently normal visual field of glaucom-atous eyes with hemifield defects. J Glaucoma. 2012; 21:318–25.
Article19. Na JH, Kook MS, Lee Y. . Detection of macular and circum-papillary structural loss in normal hemifield areas of glaucomatous eyes with localized visual field defects using spectral-domain opti-cal coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2012; 250:595–602.
Article20. Fortune B, Hardin C, Reynaud J. . Comparing optic nerve head rim width, rim area, and peripapillary retinal nerve fiber layer thick-ness to axon count in experimental glaucoma. Invest Ophthalmol Vis Sci. 2016; 57:OCT404–12.
Article21. Fortune B, Reynaud J, Hardin C. . Experimental glaucoma causes optic nerve head neural rim tissue compression: a poten-tially important mechanism of axon injury. Invest Ophthalmol Vis Sci. 2016; 57:4403–11.
Article22. Lee SH, Kim SH, Kim TW. . Reproducibility of retinal nerve fiber thickness measurements using the test-retest function of spec-tral OCT/SLO in normal and glaucomatous eyes. J Glaucoma. 2010; 19:637–42.
Article23. Mwanza JC, Chang RT, Budenz DL. . Reproducibility of peri-papillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010; 51:5724–30.
Article24. Hwang YH, Song M, Kim DW, Uhm KB. Retinal nerve fiber layer thickness measurement repeatability for cirrus HD-OCT retinal tracking system during eye movement. J Glaucoma. 2016; 25:e214–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retinal Nerve Fiber Layer-to-Disc Ratio Distinguishing Glaucoma from Nonarteritic Anterior Ischemic Optic Neuropathy
- Repeatability of Bruch’s Membrane Opening-minimum Rim Width in Age-related Macular Degeneration and Diabetic Macular Edema
- Applicability of ISNT Rule Using Bruch's Membrane Opening-based Optic Nerve Head Parameters
- Changes of Optical Coherence Tomography Parameters after Cataract Surgery in Primary Open-Angle Glaucoma Eyes
- Reproducibility of Retinal Nerve Fiber Layer Thickness Evaluation by Nerve Fiber Analyzer