Korean J Physiol Pharmacol.
2017 Jul;21(4):371-376. 10.4196/kjpp.2017.21.4.371.
Reactive oxygen species increase neuronal excitability via activation of nonspecific cation channel in rat medullary dorsal horn neurons
- Affiliations
-
- 1Department of Dental Hygiene, Gwangyang Health Science University, Gwangyang 57764, Korea.
- 2Department of Physiology, College of Medicine, Wonkwang University, Iksan 54538, Korea.
- 3Department of Oral Physiology, College of Dentistry, Wonkwang University, Iksan 54538, Korea. physio1@wonkwang.ac.kr
- KMID: 2384450
- DOI: http://doi.org/10.4196/kjpp.2017.21.4.371
Abstract
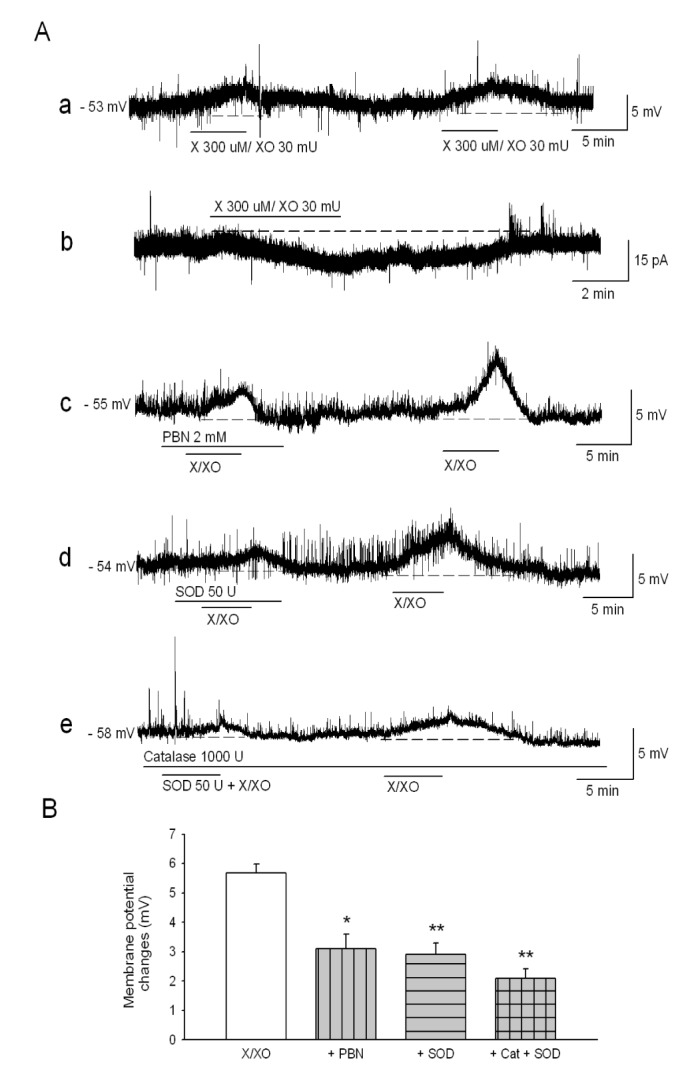
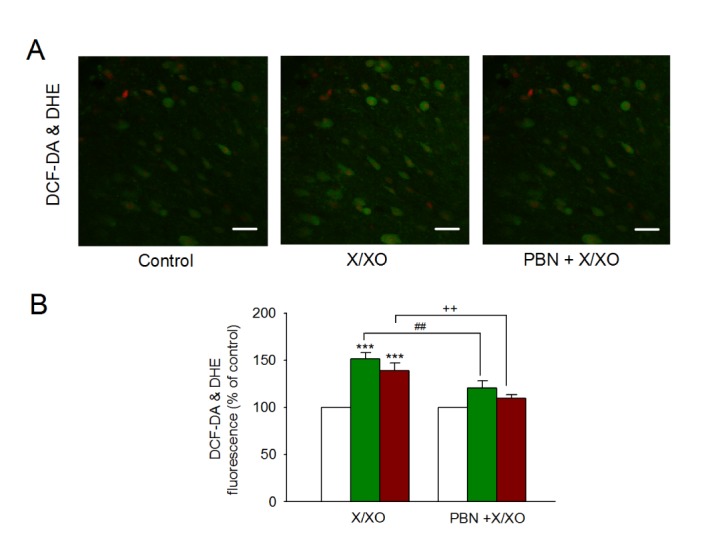
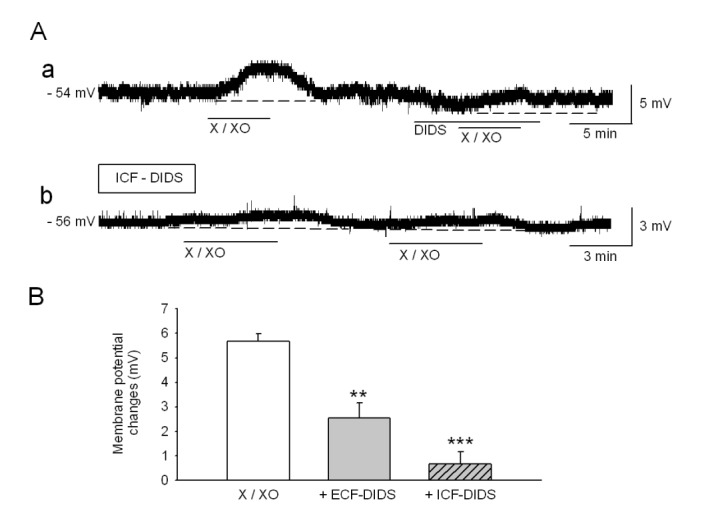
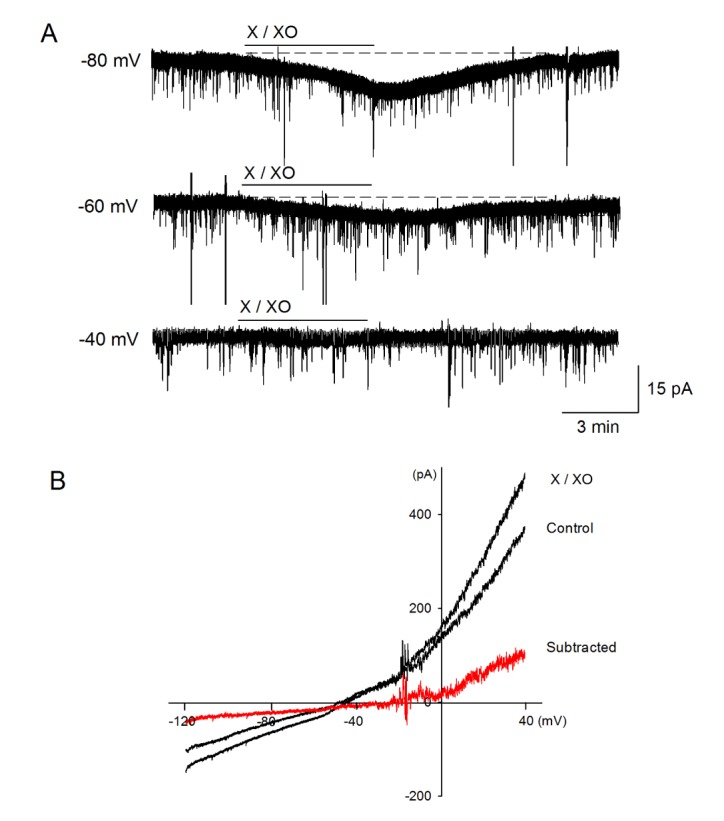
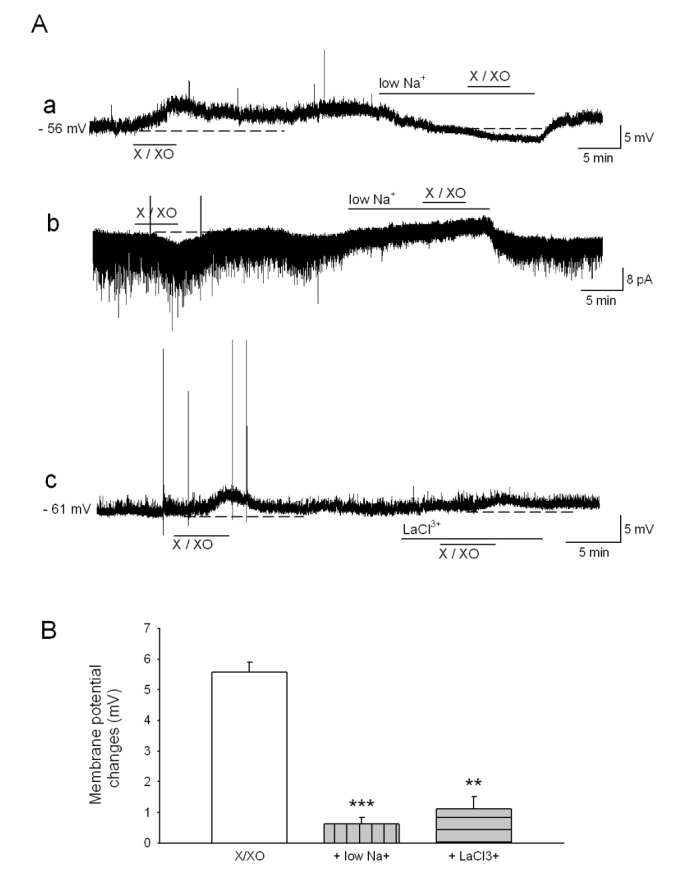
- The caudal subnucleus of the spinal trigeminal nucleus (medullary dorsal horn; MDH) receives direct inputs from small diameter primary afferent fibers that predominantly transmit nociceptive information in the orofacial region. Recent studies indicate that reactive oxygen species (ROS) is involved in persistent pain, primarily through spinal mechanisms. In this study, we aimed to investigate the role of xanthine/xanthine oxidase (X/XO) system, a known generator of superoxide anion (Oâ‚‚(·âˆ’)), on membrane excitability in the rat MDH neurons. For this, we used patch clamp recording and confocal imaging. An application of X/XO (300 µM/30 mU) induced membrane depolarization and inward currents. When slices were pretreated with ROS scavengers, such as phenyl N-tert-butylnitrone (PBN), superoxide dismutase (SOD), and catalase, X/XO-induced responses decreased. Fluorescence intensity in the DCF-DA and DHE-loaded MDH cells increased on the application of X/XO. An anion channel blocker, 4,4-diisothiocyanatostilbene-2,2-disulfonic acid (DIDS), significantly decreased X/XO-induced depolarization. X/XO elicited an inward current associated with a linear current-voltage relationship that reversed near −40 mV. X/XO-induced depolarization reduced in the presence of La³âº, a nonselective cation channel (NSCC) blocker, and by lowering the external sodium concentration, indicating that membrane depolarization and inward current are induced by influx of Na⺠ions. In conclusion, X/XO-induced ROS modulate the membrane excitability of MDH neurons, which was related to the activation of NSCC.
MeSH Terms
-
Animals
Catalase
Facial Pain
Fluorescence
Ions
Membranes
Neurons*
Oxidoreductases
Posterior Horn Cells*
Rats*
Reactive Oxygen Species*
Sodium
Spinal Cord Dorsal Horn*
Superoxide Dismutase
Superoxides
Trigeminal Nucleus, Spinal
Xanthine Oxidase
Catalase
Ions
Oxidoreductases
Reactive Oxygen Species
Sodium
Superoxide Dismutase
Superoxides
Xanthine Oxidase
Figure
Reference
-
1. Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983; 6:381–418. PMID: 6132587.
Article2. Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000; 11:57–91. PMID: 10682901.
Article3. Meller ST, Cummings CP, Traub RJ, Gebhart GF. The role of nitric oxide in the development and maintenance of the hyperalgesia produced by intraplantar injection of carrageenan in the rat. Neuroscience. 1994; 60:367–374. PMID: 8072688.
Article4. Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury: the possible role of the iron-catalyzed Haber-Weiss reaction. J Neurotrauma. 2004; 21:805–816. PMID: 15253806.
Article5. Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004; 309:869–878. PMID: 14988418.
Article6. Kim HY, Wang J, Lu Y, Chung JM, Chung K. Superoxide signaling in pain is independent of nitric oxide signaling. Neuroreport. 2009; 20:1424–1428. PMID: 19794317.
Article7. Gwak YS, Hassler SE, Hulsebosch CE. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain. 2013; 154:1699–1708. PMID: 23707296.
Article8. Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008; 138:514–524. PMID: 18375065.
Article9. Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998; 275:C1–C24. PMID: 9688830.10. Hool LC. Reactive oxygen species in cardiac signalling: from mitochondria to plasma membrane ion channels. Clin Exp Pharmacol Physiol. 2006; 33:146–151. PMID: 16445714.
Article11. Bao L, Avshalumov MV, Rice ME. Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. J Neurosci. 2005; 25:10029–10040. PMID: 16251452.
Article12. Park AR, Lee HI, Semjid D, Kim DK, Chun SW. Dual effect of exogenous nitric oxide on neuronal excitability in rat substantia gelatinosa neurons. Neural Plast. 2014; DOI: 10.1155/2014/628531.
Article13. Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, Kap O, Yumru M, Savas HA, Akyol O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007; 38:247–252. PMID: 17227736.
Article14. Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001; 31:430–439. PMID: 11498276.
Article15. Kwak KH, Han CG, Lee SH, Jeon Y, Park SS, Kim SO, Baek WY, Hong JG, Lim DG. Reactive oxygen species in rats with chronic post-ischemia pain. Acta Anaesthesiol Scand. 2009; 53:648–656. PMID: 19419360.
Article16. Sato E, Mokudai T, Niwano Y, Kohno M. Kinetic analysis of reactive oxygen species generated by the in vitro reconstituted NADPH oxidase and xanthine oxidase systems. J Biochem. 2011; 150:173–181. PMID: 21572100.
Article17. Brzezinska AK, Lohr N, Chilian WM. Electrophysiological effects of O2·− on the plasma membrane in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2005; 289:H2379–H2386. PMID: 15964927.18. Callaghan MJ, Ceradini DJ, Gurtner GC. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal. 2005; 7:1476–1482. PMID: 16356110.
Article19. Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007; 292:R18–R36. PMID: 16917020.
Article20. Loffredo L, Marcoccia A, Pignatelli P, Andreozzi P, Borgia MC, Cangemi R, Chiarotti F, Violi F. Oxidative-stress-mediated arterial dysfunction in patients with peripheral arterial disease. Eur Heart J. 2007; 28:608–612. PMID: 17298965.
Article21. Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009; 11:2481–2504. PMID: 19309263.
Article22. Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B. Trigeminal pain transmission requires reactive oxygen species production. Brain Res. 2005; 1050:72–78. PMID: 15979588.
Article23. Kallenborn-Gerhardt W, Schröder K, Geisslinger G, Schmidtko A. NOXious signaling in pain processing. Pharmacol Ther. 2013; 137:309–317. PMID: 23146925.
Article24. Shanker G, Aschner JL, Syversen T, Aschner M. Free radical formation in cerebral cortical astrocytes in culture induced by methylmercury. Brain Res Mol Brain Res. 2004; 128:48–57. PMID: 15337317.
Article25. Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2',7'-dichlorodihydrofluor escein diacetate, 5(and 6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999; 27:146–159. PMID: 10443931.26. Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996; 16:1324–1336. PMID: 8778284.
Article27. Sgherri CLM, Pinzino C, Navari-Izzo F. Sunflower seedlings subjected to increasing stress by water deficit: Changes in O2·−production related to the composition of thylakoid membranes. Physiol Plant. 1996; 96:446–452.28. Lynch RE, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978; 253:4697–4699. PMID: 207707.
Article29. Ikebuchi Y, Masumoto N, Tasaka K, Koike K, Kasahara K, Miyake A, Tanizawa O. Superoxide anion increases intracellular pH, intracellular free calcium, and arachidonate release in human amnion cells. J Biol Chem. 1991; 266:13233–13237. PMID: 1649184.
Article30. Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003; 278:5557–5563. PMID: 12482755.
Article31. Granville DJ, Gottlieb RA. The mitochondrial voltage-dependent anion channel (VDAC) as a therapeutic target for initiating cell death. Curr Med Chem. 2003; 10:1527–1533. PMID: 12871124.
Article32. Koliwad SK, Kunze DL, Elliott SJ. Oxidant stress activates a non-selective cation channel responsible for membrane depolarization in calf vascular endothelial cells. J Physiol. 1996; 491:1–12. PMID: 9011602.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Action of Mitochondrial Substrates on Neuronal Excitability in Rat Substantia Gelatinosa Neurons
- Effects of Mitochondrial Reactive Oxygen Species on Neuronal Excitability in Rat Spinal Substantia Gelatinosa Neurons
- Effects of Reactive Oxygen Species and Nitrogen Species on the Excitability of Spinal Substantia Gelatinosa Neurons
- Effects of NaOCl on the Intracellular Calcium Concentration in Rat Dorsal Root Ganglion Neurons
- Reactive Oxygen Species and Nitrogen Species Differentially Regulate Neuronal Excitability in Rat Spinal Substantia Gelatinosa Neurons