Endocrinol Metab.
2014 Dec;29(4):536-544. 10.3803/EnM.2014.29.4.536.
Expression of Glucagon-Like Peptide-1 Receptor in Papillary Thyroid Carcinoma and Its Clinicopathologic Significance
- Affiliations
-
- 1Department of Pathology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
- 2Department of Endocrinology and Metabolism, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea. mir316@naver.com
- KMID: 2384252
- DOI: http://doi.org/10.3803/EnM.2014.29.4.536
Abstract
- BACKGROUND
Incretin-based therapies are rapidly becoming one of the main glycemic control strategies in diabetes. Considering the large numbers of papillary thyroid carcinomas (PTCs) and possible effects of glucagon-like peptide-1 (GLP-1) on cell proliferation, the expression of GLP-1 receptor (GLP-1R) in PTC is likely to have clinical significance. We performed this study to evaluate the expression of GLP-1R in PTC and the clinical meaning of GLP-1R expression in PTC.
METHODS
Fifty-six cases of PTC, four cases of medullary thyroid cancer (MTC), seven cases of nodular hyperplasia and 56 normal thyroid tissue samples were selected for immunostaining for GLP-1R. Clinical parameters were obtained by retrospective review of medical records.
RESULTS
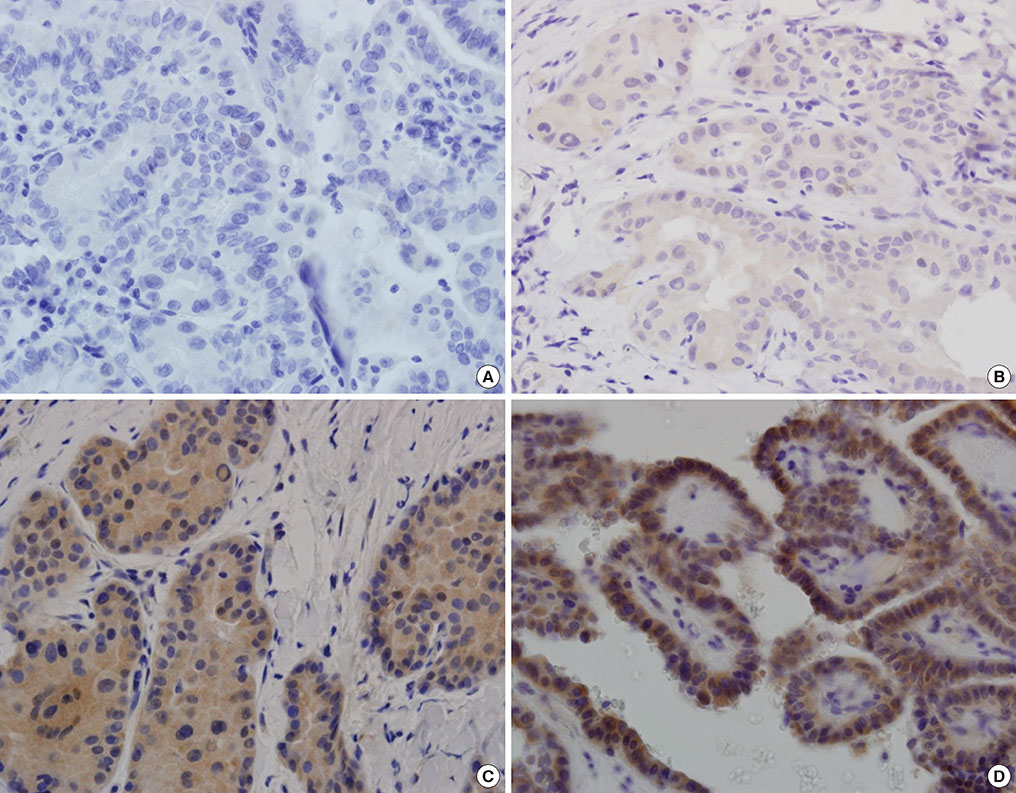
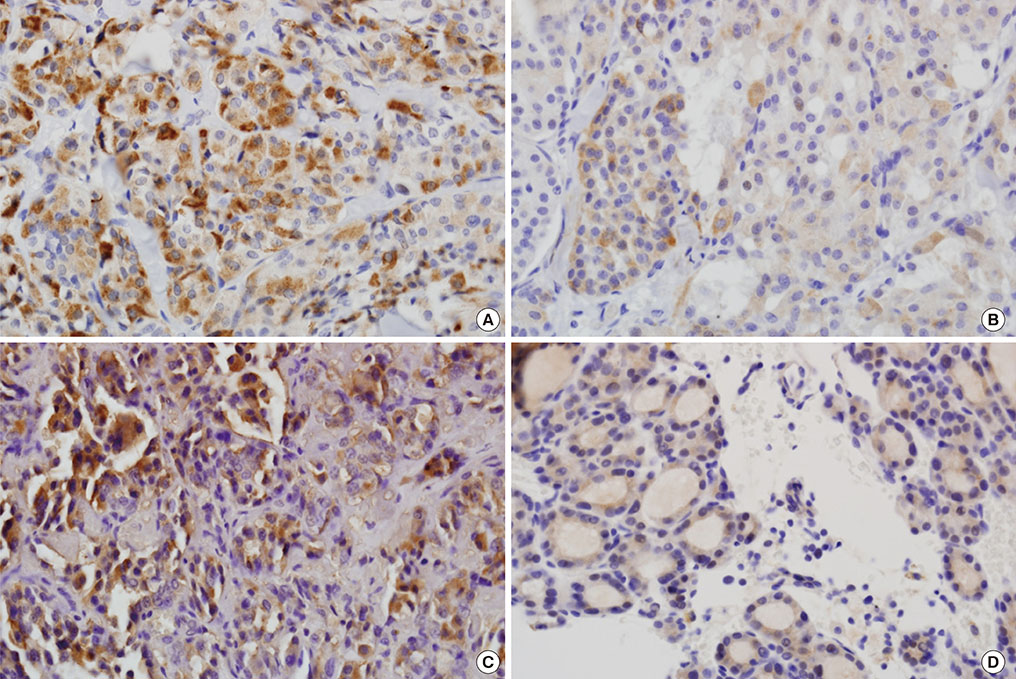
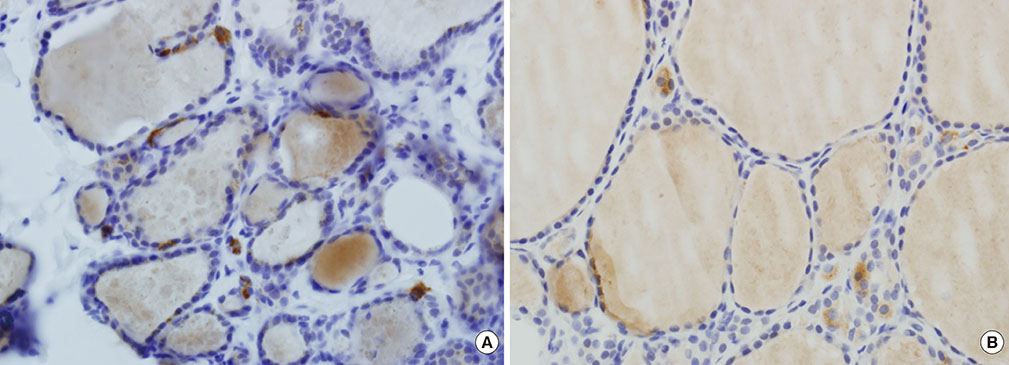
Immunohistochemical staining for GLP-1R showed immunoreactivity in 18 of 56 cases of PTC (32.1%). All four cases of MTC exhibited cytoplasmic GLP-1R expression. Nodular hyperplasia exhibited immunoreactivity in two of seven cases (28.6%). All normal thyroid follicular cells showed negative immunoreactivity. In univariable and multivariable analyses, tumor multifocality was negatively correlated with GLP-1R expression. Extrathyroidal extension showed positive association with GLP-1R expression that was almost significant. Sex, age, tumor size, and lymph node metastasis were not significantly associated with GLP-1R expression.
CONCLUSION
Some parts of PTC tissues express GLP-1R, and GLP-1R expression in PTC was negatively correlated with tumor multifocality. The long-term influence of pharmacologically increased GLP-1 on thyroid follicular cells and development and progression of tumors originating from thyroid follicular cells should be investigated.
Keyword
MeSH Terms
Figure
Reference
-
1. American Diabetes Association. Standards of medical care in diabetes: 2014. Diabetes Care. 2014; 37:Suppl 1. S14–S80.2. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH. American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013; 19:327–336.3. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–1589.4. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005; 353:2643–2653.5. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.6. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572.7. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–139.8. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006; 368:1696–1705.9. Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care. 2006; 29:471.10. Cure P, Pileggi A, Alejandro R. Exenatide and rare adverse events. N Engl J Med. 2008; 358:1969–1970.11. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009; 374:39–47.12. Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000; 141:4600–4605.13. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011; 141:150–156.14. Hegedus L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab. 2011; 96:853–860.15. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369:1317–1326.16. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013; 369:1327–1335.17. Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-like-peptide-1 receptor expression in normal and diseased human thyroid and pancreas. Mod Pathol. 2014; 09. 12. Epub. DOI: http://dx.doi.org/10.1038/modpathol.2014.113.18. Crespel A, De Boisvilliers F, Gros L, Kervran A. Effects of glucagon and glucagon-like peptide-1-(7-36) amide on C cells from rat thyroid and medullary thyroid carcinoma CA-77 cell line. Endocrinology. 1996; 137:3674–3680.19. Lamari Y, Boissard C, Moukhtar MS, Jullienne A, Rosselin G, Garel JM. Expression of glucagon-like peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagon-like peptide 1. FEBS Lett. 1996; 393:248–252.20. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010; 151:1473–1486.21. Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology. 2011; 94:291–301.22. Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab. 2012; 97:121–131.23. Korner M, Stockli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007; 48:736–743.24. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.25. Schlumberger M, Pacini F, Wiersinga WM, Toft A, Smit JW, Sanchez Franco F, Lind P, Limbert E, Jarzab B, Jamar F, Duntas L, Cohen O, Berg G. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol. 2004; 151:539–548.26. Cross S, Wei JP, Kim S, Brams DM. Selective surgery and adjuvant therapy based on risk classifications of well-differentiated thyroid cancer. J Surg Oncol. 2006; 94:678–682.27. Ricci JA, Alfonso AE. Multifocal micropapillary thyroid cancer: a new indication for total thyroidectomy? Am Surg. 2012; 78:1211–1214.28. Kim HJ, Sohn SY, Jang HW, Kim SW, Chung JH. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg. 2013; 37:376–384.29. Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, Huang T. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol. 2013; 20:746–752.30. Schindler AM, van Melle G, Evequoz B, Scazziga B. Prognostic factors in papillary carcinoma of the thyroid. Cancer. 1991; 68:324–330.31. Ross DS, Litofsky D, Ain KB, Bigos T, Brierley JD, Cooper DS, Haugen BR, Jonklaas J, Ladenson PW, Magner J, Robbins J, Skarulis MC, Steward DL, Maxon HR, Sherman SI. Recurrence after treatment of micropapillary thyroid cancer. Thyroid. 2009; 19:1043–1048.32. Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol. 2015; 22:125–131.33. Dalle S, Burcelin R, Gourdy P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic beta-cell impairments in type 2 diabetes. Cell Signal. 2013; 25:570–579.34. Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide 1 (7-36) amide stimulates exocytosis in human pancreatic beta-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes. 1998; 47:57–65.35. Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2'-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem. 2003; 278:8279–8285.36. Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf). 2012; 204:277–287.37. Madsen LW, Knauf JA, Gotfredsen C, Pilling A, Sjogren I, Andersen S, Andersen L, de Boer AS, Manova K, Barlas A, Vundavalli S, Nyborg NC, Knudsen LB, Moelck AM, Fagin JA. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012; 153:1538–1547.38. Brubaker PL, Drucker DJ. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004; 145:2653–2659.39. Koehler JA, Kain T, Drucker DJ. Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology. 2011; 152:3362–3372.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Expression of Glucagon-Like Peptide-1 Receptor in Papillary Thyroid Carcinoma and Its Clinicopathologic Significance (Endocrinol Metab 2014;29:536-44, Min Jung Jung et al.)
- Response: Expression of Glucagon-Like Peptide-1 Receptor in Papillary Thyroid Carcinoma and Its Clinicopathologic Significance (Endocrinol Metab 2014;29:536-44, Min Jung Jung et al.)
- Concurrent Medullay and Papillary Carcinoma of the Thyroid
- Glucagon-like Peptide-1 Analogue and Dipeptidyl Peptidase-IV Inhibitors
- Glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes