J Gastric Cancer.
2017 Jun;17(2):120-131. 10.5230/jgc.2017.17.e15.
Effect of a Proton Pump Inhibitor on Tumor Bleeding Prevention in Unresectable Gastric Cancer Patients: a Double-Blind, Randomized, Placebo-Controlled Trial
- Affiliations
-
- 1Center for Gastric Cancer, National Cancer Center, Goyang, Korea. cij1224@ncc.re.kr
- 2Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Pusan National University School of Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- 4Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea.
- 5Biometric Research Branch, Research Institute for National Cancer Control & Evaluation, National Cancer Center, Goyang, Korea.
- KMID: 2383735
- DOI: http://doi.org/10.5230/jgc.2017.17.e15
Abstract
- PURPOSE
Tumor bleeding is a major complication in inoperable gastric cancer. The study aim was to investigate the effects of proton pump inhibitor (PPI) treatment for the prevention of gastric tumor bleeding.
MATERIALS AND METHODS
This study was a prospective double-blind, randomized, placebo-controlled trial. Patients with inoperable gastric cancer were randomly assigned to receive oral lansoprazole (30 mg) or placebo daily. The primary endpoint was the occurrence of tumor bleeding, and the secondary endpoints were transfusion requirement and overall survival (OS).
RESULTS
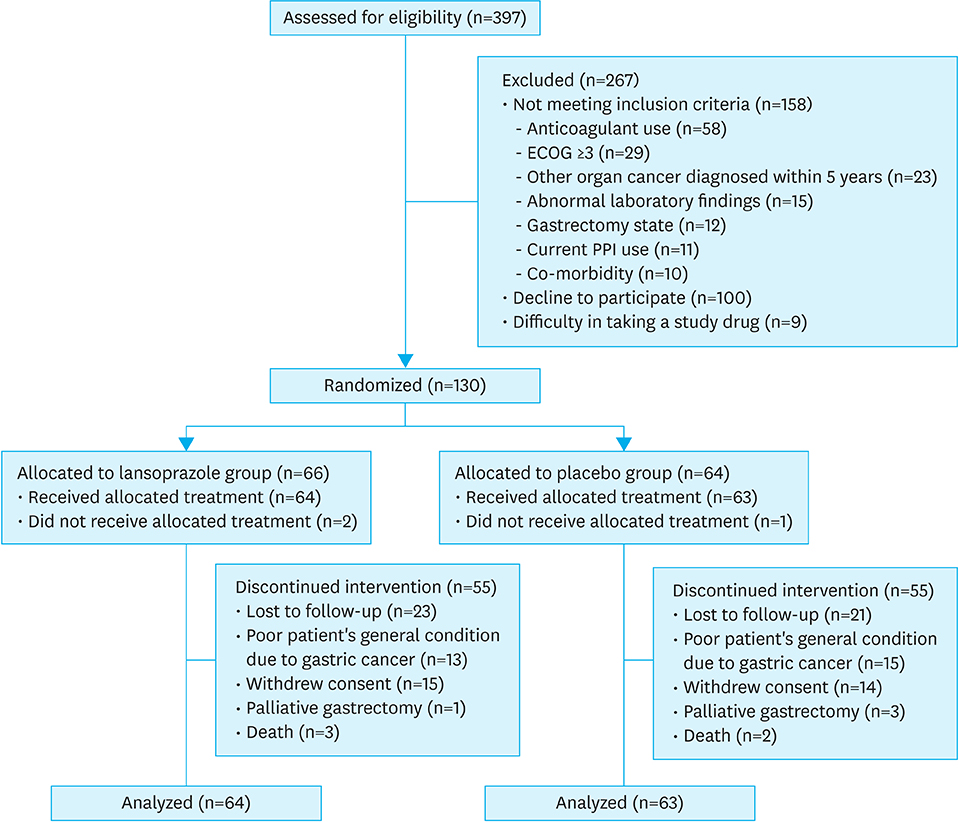
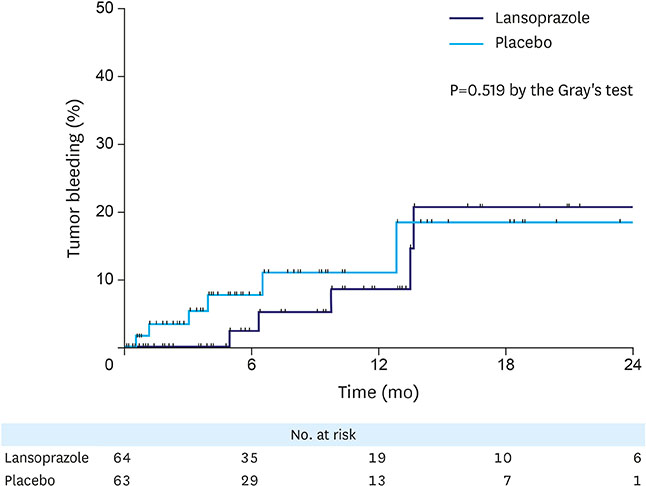
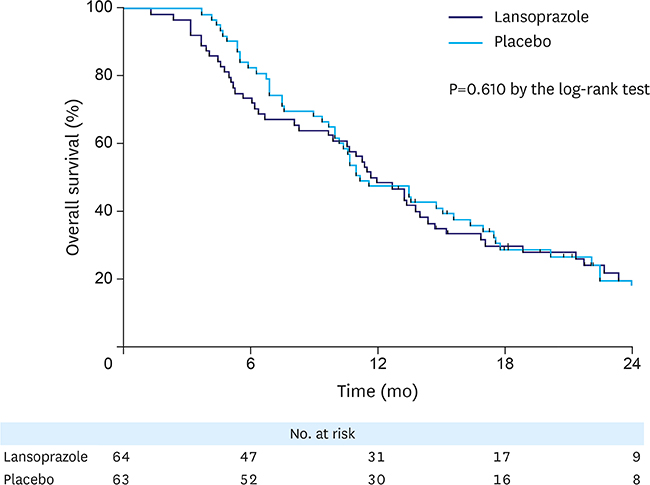
This study initially planned to enroll 394 patients, but prematurely ended due to low recruitment rate. Overall, 127 patients were included in the analyses: 64 in the lansoprazole group and 63 in the placebo group. During the median follow-up of 6.4 months, tumor bleeding rates were 7.8% and 9.5%, in the lansoprazole and placebo groups, respectively, with the cumulative bleeding incidence not statistically different between the groups (P=0.515, Gray's test). However, during the initial 4 months, 4 placebo-treated patients developed tumor bleeding, whereas there were no bleeding events in the lansoprazole-treated patients (P=0.041, Gray's test). There was no difference in the proportion of patients who required transfusion between the groups. The OS between the lansoprazole (11.7 months) and the placebo (11.0 months) groups was not statistically different (P=0.610). Study drug-related serious adverse event or bleeding-related death did not occur.
CONCLUSIONS
Treating patients with inoperable gastric cancer with lansoprazole did not significantly reduce the incidence of tumor bleeding. However, further studies are needed to evaluate whether lansoprazole can prevent tumor bleeding during earlier phases of chemotherapy (ClinicalTrial.gov, identifier No. NCT02150447).
MeSH Terms
Figure
Reference
-
1. Savides TJ, Jensen DM, Cohen J, Randall GM, Kovacs TO, Pelayo E, et al. Severe upper gastrointestinal tumor bleeding: endoscopic findings, treatment, and outcome. Endoscopy. 1996; 28:244–248.2. Sheibani S, Kim JJ, Chen B, Park S, Saberi B, Keyashian K, et al. Natural history of acute upper GI bleeding due to tumours: short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther. 2013; 38:144–150.3. Loftus EV Jr, Alexander GL, Ahlquist DA, Balm RK. Endoscopic treatment of major bleeding from advanced gastroduodenal malignant lesions. Mayo Clin Proc. 1994; 69:736–740.4. Kim YI, Choi IJ, Cho SJ, Lee JY, Kim CG, Kim MJ, et al. Outcome of endoscopic therapy for cancer bleeding in patients with unresectable gastric cancer. J Gastroenterol Hepatol. 2013; 28:1489–1495.5. Koh KH, Kim K, Kwon DH, Chung BS, Sohn JY, Ahn DS, et al. The successful endoscopic hemostasis factors in bleeding from advanced gastric cancer. Gastric Cancer. 2013; 16:397–403.6. Neumann I, Letelier LM, Rada G, Claro JC, Martin J, Howden CW, et al. Comparison of different regimens of proton pump inhibitors for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2013; CD007999.7. Lau JY, Leung WK, Wu JC, Chan FK, Wong VW, Chiu PW, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007; 356:1631–1640.8. Lau JY, Sung JJ, Lee KK, Yung MY, Wong SK, Wu JC, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000; 343:310–316.9. Chan FK, Wong VW, Suen BY, Wu JC, Ching JY, Hung LC, et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet. 2007; 369:1621–1626.10. Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002; 346:2033–2038.11. Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015; 47:a1–a46.12. Hwang JH, Fisher DA, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012; 75:1132–1138.13. Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974; 2:394–397.14. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988; 16:1141–1154.15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94:496–509.16. Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis of proton pump inhibitor therapy in peptic ulcer bleeding. BMJ. 2005; 330:568.17. Lanas A, García-Rodríguez LA, Arroyo MT, Bujanda L, Gomollón F, Forné M, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007; 102:507–515.18. Kim YI, Choi IJ. Endoscopic management of tumor bleeding from inoperable gastric cancer. Clin Endosc. 2015; 48:121–127.19. Laine L, Shah A, Bemanian S. Intragastric pH with oral vs intravenous bolus plus infusion proton-pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology. 2008; 134:1836–1841.20. Green FW Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978; 74:38–43.21. Sakita T, Oguro Y, Takasu S, Fukutomi H, Miwa T. Observations on the healing of ulcerations in early gastric cancer. The life cycle of the malignant ulcer. Gastroenterology. 1971; 60:835–839.22. Myung YS, Hong SJ, Han JP, Park KW, Ko BM, Lee MS. Effects of administration of a proton pump inhibitor before endoscopic submucosal dissection for differentiated early gastric cancer with ulcer. Gastric Cancer. 2017; 20:200–206.23. Imbesi JJ, Kurtz RC. A multidisciplinary approach to gastrointestinal bleeding in cancer patients. J Support Oncol. 2005; 3:101–110.24. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015; 372:1832–1843.25. Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010; CD004064.26. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697.27. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011; 29:3968–3976.28. Suh M, Choi KS, Park B, Lee YY, Jun JK, Lee DH, et al. Trends in cancer screening rates among Korean men and women: results of the Korean National Cancer Screening Survey, 2004–2013. Cancer Res Treat. 2016; 48:1–10.29. Kim YG, Kong SH, Oh SY, Lee KG, Suh YS, Yang JY, et al. Effects of screening on gastric cancer management: comparative analysis of the results in 2006 and in 2011. J Gastric Cancer. 2014; 14:129–134.30. Kim HS, Lee H, Jeung HC, Noh SH, Chung HC, Roh JK, et al. Advanced detection of recent changing trends in gastric cancer survival: up-to-date comparison by period analysis. Jpn J Clin Oncol. 2011; 41:1344–1350.31. Kim SM, Park SH. Chemotherapy beyond second-line in advanced gastric cancer. World J Gastroenterol. 2015; 21:8811–8816.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the Addition of Mosapride to Gastroesophageal Reflux Disease Patients on Proton Pump Inhibitor: A Prospective Randomized, Double-blind Study
- Endoscopic Management of Tumor Bleeding from Inoperable Gastric Cancer
- Drug Interaction between Proton Pump Inhibitors and Clopidogrel: Safe Perspective
- Do Histamine-2 Receptor Antagonists and Proton Pump Inhibitors Really Have No Effect on the Gastric Emptying Rate?
- Proton Pump Inhibitors with Clopidogrel: Is There Benefit or Harm?