Ann Dermatol.
2016 Oct;28(5):548-554. 10.5021/ad.2016.28.5.548.
Expression of Phosphatase and Tensin Homologue, phospho-Akt, and p53 in Acral Benign and Malignant Melanocytic Neoplasms (Benign Nevi, Dysplastic Nevi, and Acral Melanomas)
- Affiliations
-
- 1Department of Dermatology, Ewha Womans University School of Medicine, Seoul, Korea. uwon313@ewha.ac.kr
- 2Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2382877
- DOI: http://doi.org/10.5021/ad.2016.28.5.548
Abstract
- BACKGROUND
The role of the phosphatidylinositol-3 kinase signaling pathway in the development of acral melanoma has recently gained evidence. Phosphatase and tensin homologue (PTEN), one of the key molecules in the pathway, acts as a tumor suppressor through either an Akt-dependent or Akt-independent pathway. Akt accelerates degradation of p53.
OBJECTIVE
We assessed the expression of PTEN, phospho-Akt (p-Akt), and p53 by immunohistochemistry in benign acral nevi, acral dysplastic nevi, and acral melanomas in the radial growth phase and with a vertical growth component.
METHODS
Ten specimens in each group were included. Paraffin-embedded specimens were immunostained with antibodies for PTEN, p-Akt, and p53. We scored both the staining intensity and the proportion of positive cells. The final score was calculated by multiplying the intensity score by the proportion score.
RESULTS
All specimens of benign acral nevi except one showed some degree of PTEN-negative cells. The numbers of p-Akt and p53-positive cells were higher in acral dysplastic nevi and melanoma than in benign nevi. P-Akt scores were 1.7, 1.8, 2.6, and 4.4, and p53 scores were 2.0, 2.1, 3.8, and 4.1 in each group. PTEN and p-Akt scores in advanced acral melanoma were higher than in the other neoplasms.
CONCLUSION
The expression of PTEN was decreased and the expression of p-Akt was increased in acral melanoma, especially in advanced cases. The PTEN-induced pathway appears to affect the late stage of melanomagenesis. Altered expression of p-Akt is thought to be due to secondary changes following the loss of PTEN.
Keyword
- Acral; Akt; Melanocytic; p53; PTEN
MeSH Terms
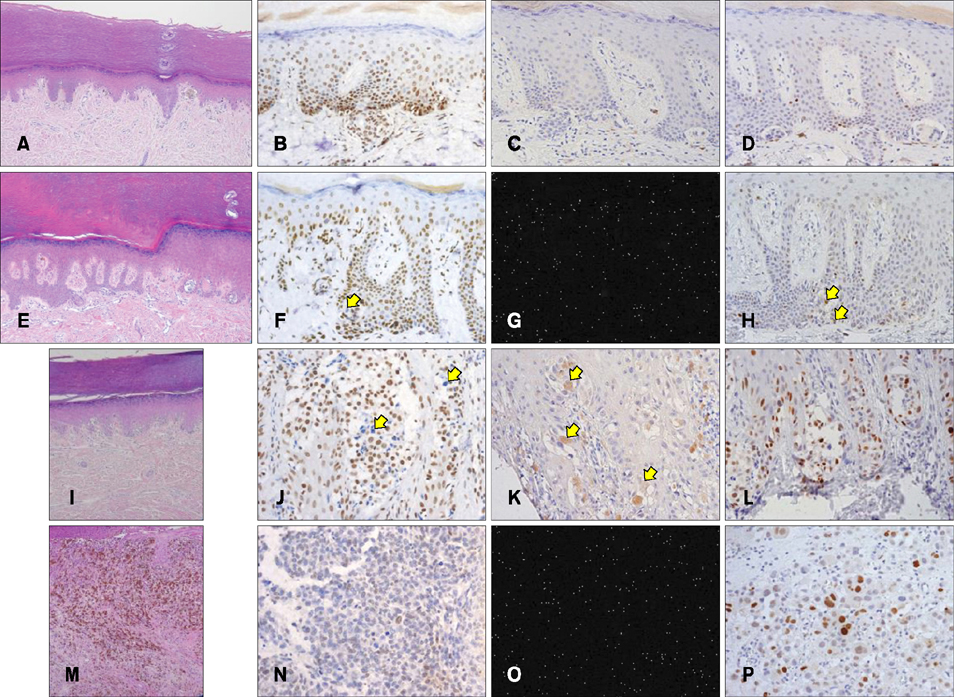
Figure
Reference
-
1. Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005; 353:2135–2147.
Article2. Broekaert SM, Roy R, Okamoto I, van den Oord J, Bauer J, Garbe C, et al. Genetic and morphologic features for melanoma classification. Pigment Cell Melanoma Res. 2010; 23:763–770.
Article3. Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007; 28:1379–1386.
Article4. Saraiva VS, Caissie AL, Segal L, Edelstein C, Burnier MN Jr. Immunohistochemical expression of phospho-Akt in uveal melanoma. Melanoma Res. 2005; 15:245–250.
Article5. Abraham AG, O'Neill E. PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans. 2014; 42:798–803.
Article6. Ministry of Health and Welfare. Annual report of cancer statistics in Korea. Sejong: Ministry of Health and Welfare;2002. 2006. 2010.7. Kim SY, Kim JS, Park K, Oh CW, Shin JH, Kang HY, et al. Epidermal and adnexal nevi and tumors. Textbook of dermatology. 6th ed. Seoul: Daehan Medical Publishing Co.;2014. p. 804.8. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417:949–954.
Article9. Goydos JS, Mann B, Kim HJ, Gabriel EM, Alsina J, Germino FJ, et al. Detection of B-RAF and N-RAS mutations in human melanoma. J Am Coll Surg. 2005; 200:362–370.
Article10. Bastian BC, Kashani-Sabet M, Hamm H, Godfrey T, Moore DH 2nd, Bröcker EB, et al. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res. 2000; 60:1968–1973.11. Zebary A, Omholt K, Vassilaki I, Höiom V, Lindén D, Viberg L, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013; 72:284–289.
Article12. Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004; 22:2954–2963.
Article13. Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003; 3:117–130.
Article14. Trotman LC, Pandolfi PP. PTEN and p53: who will get the upper hand? Cancer Cell. 2003; 3:97–99.
Article15. Reifenberger J, Wolter M, Boström J, Büschges R, Schulte KW, Megahed M, et al. Allelic losses on chromosome arm 10q and mutation of the PTEN (MMAC1) tumour suppressor gene in primary and metastatic malignant melanomas. Virchows Arch. 2000; 436:487–493.
Article16. Shull AY, Latham-Schwark A, Ramasamy P, Leskoske K, Oroian D, Birtwistle MR, et al. Novel somatic mutations to PI3K pathway genes in metastatic melanoma. PLoS One. 2012; 7:e43369.
Article17. Whiteman DC, Zhou XP, Cummings MC, Pavey S, Hayward NK, Eng C. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer. 2002; 99:63–67.
Article18. Tsao H, Mihm MC Jr, Sheehan C. PTEN expression in normal skin, acquired melanocytic nevi, and cutaneous melanoma. J Am Acad Dermatol. 2003; 49:865–872.
Article19. Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008; 99:1265–1268.
Article20. Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005; 23:1473–1482.
Article21. Shanesmith RP, Smart C, Cassarino DS. Tissue microarray analysis of ezrin, KBA.62, CD166, nestin, and p-Akt in melanoma versus banal and atypical nevi, and nonmelanocytic lesions. Am J Dermatopathol. 2011; 33:663–668.
Article22. Ragnarsson-Olding BK, Karsberg S, Platz A, Ringborg UK. Mutations in the TP53 gene in human malignant melanomas derived from sun-exposed skin and unexposed mucosal membranes. Melanoma Res. 2002; 12:453–463.
Article23. Talve L, Kainu J, Collan Y, Ekfors T. Immunohistochemical expression of p53 protein, mitotic index and nuclear morphometry in primary malignant melanoma of the skin. Pathol Res Pract. 1996; 192:825–833.
Article24. McGregor JM, Yu CC, Dublin EA, Barnes DM, Levison DA, MacDonald DM. p53 immunoreactivity in human malignant melanoma and dysplastic naevi. Br J Dermatol. 1993; 128:606–611.
Article25. Kanoko M, Ueda M, Nagano T, Ichihashi M. Expression of p53 protein in melanoma progression. J Dermatol Sci. 1996; 12:97–103.
Article26. Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, Bornemann A, et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med. 2013; 2:76–85.
Article27. Mao M, Tian F, Mariadason JM, Tsao CC, Lemos R Jr, Dayyani F, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2013; 19:657–667.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dermoscopic Patterns of Acral Melanocytic Nevi in Koreans
- Immunohistochemical Double Staining of Ki-67/Melan-A in Melanocytic Nevi and Malignant Melanomas
- Immunohistochemical Labeling of Melanocytic Differentiation Antibodies in Melanocytic Nevi and Malignant Melanomas
- An Immunohistochemical Study of pRb, p16, p53 and p21 Protein Expression in Malignant Melanoma
- p53 Expression in Malignant Melanoma and Melanocytic Nevus