Int J Stem Cells.
2015 Nov;8(2):228-234. 10.15283/ijsc.2015.8.2.228.
FGF8 is Essential for Functionality of Induced Neural Precursor Cell-derived Dopaminergic Neurons
- Affiliations
-
- 1Graduate School of Biomedical Science and Engineering, College of Medicine, Hanyang University, Seoul, Korea. chshpark@hanyang.ac.kr
- 2Hanyang Biomedical Research Institute, College of Medicine, Hanyang University, Seoul, Korea.
- 3Department of Microbiology, College of Medicine, Hanyang University, Seoul, Korea.
- KMID: 2380817
- DOI: http://doi.org/10.15283/ijsc.2015.8.2.228
Abstract
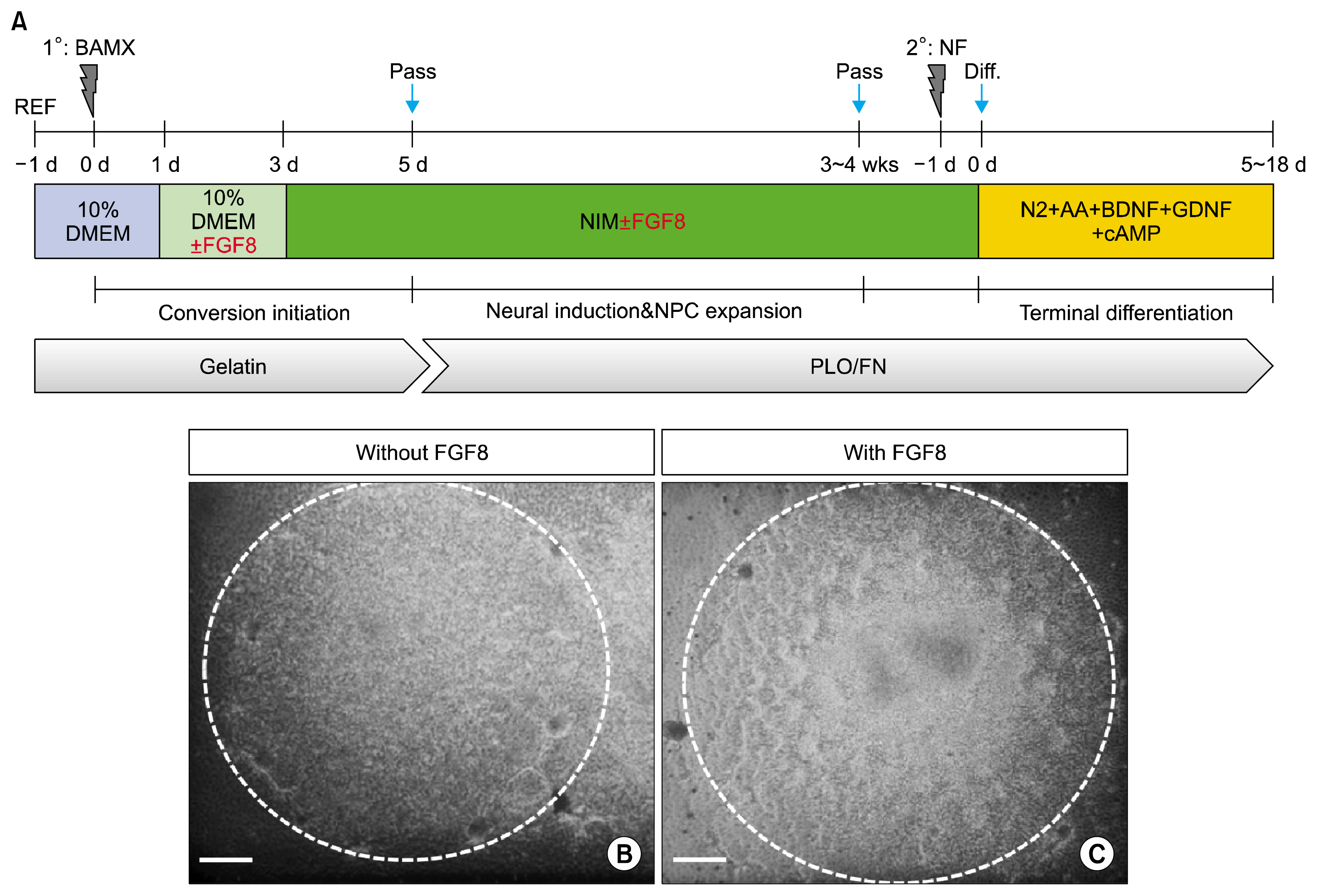
- Induced neural precursor cells (iNPCs) are one source of transplantable dopaminergic neurons used in cell therapy for Parkinson's disease. In the present study, we demonstrate that iNPCs can be generated by transducing Brn2, Ascl1, Myt1L and Bcl-xL in a culture supplemented with several mitogens and subsequently can be differentiated to dopaminergic neurons (DA). However, studies have shown that iDA and/or iNPC-derived DA neurons using various conversion protocols have low efficiency. Here, we show that early exposure of FGF8 to fibroblasts efficiently improves differentiation of DA neurons. So our study demonstrates that FGF8 is a critical factor for generation of iNPC-derived DA neurons.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Hybrid Nanofiber Scaffold-Based Direct Conversion of Neural Precursor Cells/Dopamine Neurons
Mi-Sun Lim, Seung Hwan Ko, Min Sung Kim, Byungjun Lee, Ho-Sup Jung, Keesung Kim, Chang-Hwan Park
Int J Stem Cells. 2019;12(2):340-346. doi: 10.15283/ijsc18123.
Reference
-
References
1. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010; 463:1035–1041. DOI: 10.1038/nature08797. PMID: 20107439. PMCID: 2829121.
Article2. Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011; 476:224–227. DOI: 10.1038/nature10284. PMID: 21725324.
Article3. Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Björklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci USA. 2011; 108:10343–10348. DOI: 10.1073/pnas.1105135108. PMID: 21646515. PMCID: 3121829.
Article4. Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA. 2011; 108:7838–7843. DOI: 10.1073/pnas.1103113108. PMID: 21521790. PMCID: 3093517.
Article5. Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, Brüstle O, Edenhofer F. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012; 10:473–479. DOI: 10.1016/j.stem.2012.03.003. PMID: 22445518.
Article6. Sheng C, Zheng Q, Wu J, Xu Z, Sang L, Wang L, Guo C, Zhu W, Tong M, Liu L, Li W, Liu ZH, Zhao XY, Wang L, Chen Z, Zhou Q. Generation of dopaminergic neurons directly from mouse fibroblasts and fibroblast-derived neural progenitors. Cell Res. 2012; 22:769–772. DOI: 10.1038/cr.2012.32. PMID: 22370632. PMCID: 3317566.
Article7. Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, Lengner CJ, Chung CY, Dawlaty MM, Tsai LH, Jaenisch R. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011; 9:413–419. DOI: 10.1016/j.stem.2011.09.011. PMID: 22019014. PMCID: 3210333.
Article8. Liu X, Li F, Stubblefield EA, Blanchard B, Richards TL, Larson GA, He Y, Huang Q, Tan AC, Zhang D, Benke TA, Sladek JR, Zahniser NR, Li CY. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res. 2012; 22:321–332. DOI: 10.1038/cr.2011.181. PMCID: 3271588.
Article9. Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995; 121:439–451. PMID: 7768185.
Article10. Partanen J. FGF signalling pathways in development of the midbrain and anterior hindbrain. J Neurochem. 2007; 101:1185–1193. DOI: 10.1111/j.1471-4159.2007.04463.x. PMID: 17326764.
Article11. Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996; 271:15292–15297. DOI: 10.1074/jbc.271.25.15292. PMID: 8663044.
Article12. Tsang M, Dawid IB. Promotion and attenuation of FGF signaling through the Ras-MAPK pathway. Sci STKE. 2004; 2004:pe17. PMID: 15082862.
Article13. Lunn JS, Fishwick KJ, Halley PA, Storey KG. A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Dev Biol. 2007; 302:536–552. DOI: 10.1016/j.ydbio.2006.10.014.
Article14. Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000; 18:675–679. DOI: 10.1038/76536. PMID: 10835609.
Article15. Tanaka A, Kamiakito T, Hakamata Y, Fujii A, Kuriki K, Fukayama M. Extensive neuronal localization and neurotrophic function of fibroblast growth factor 8 in the nervous system. Brain Res. 2001; 912:105–115. DOI: 10.1016/S0006-8993(01)02726-3. PMID: 11532426.
Article16. Park CH, Kang JS, Yoon EH, Shim JW, Suh-Kim H, Lee SH. Proneural bHLH neurogenin 2 differentially regulates Nurr1-induced dopamine neuron differentiation in rat and mouse neural precursor cells in vitro. FEBS Lett. 2008; 582:537–542. DOI: 10.1016/j.febslet.2008.01.018. PMID: 18242186.
Article17. Lim MS, Chang MY, Kim SM, Yi SH, Suh-Kim H, Jung SJ, Kim MJ, Kim JH, Lee YS, Lee SY, Kim DW, Lee SH, Park CH. Generation of dopamine neurons from rodent fibroblasts through the expandable neural precursor cell stage. J Biol Chem. 2015; 290:17401–17414. DOI: 10.1074/jbc.M114.629808. PMID: 26023233. PMCID: 4498077.
Article18. He XB, Yi SH, Rhee YH, Kim H, Han YM, Lee SH, Lee H, Park CH, Lee YS, Richardson E, Kim BW, Lee SH. Prolonged membrane depolarization enhances midbrain dopamine neuron differentiation via epigenetic histone modifications. Stem Cells. 2011; 29:1861–1873. DOI: 10.1002/stem.739. PMID: 21922608.
Article19. Hajihosseini MK, Dickson C. A subset of fibroblast growth factors (Fgfs) promote survival, but Fgf-8b specifically promotes astroglial differentiation of rat cortical precursor cells. Mol Cell Neurosci. 1999; 14:468–485. DOI: 10.1006/mcne.1999.0800.
Article20. Cooper O, Hargus G, Deleidi M, Blak A, Osborn T, Marlow E, Lee K, Levy A, Perez-Torres E, Yow A, Isacson O. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010; 45:258–266. DOI: 10.1016/j.mcn.2010.06.017. PMID: 20603216. PMCID: 2945816.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Embryonic Stem Cell-derived Dopaminergic Neurons and E12 Mesencephalic Neuronal-precursor Derived Dopaminergic Neurons as a Source for Transplantation in Parkinsonian Rats
- Direct Differentiation of Adult Ocular Progenitors into Striatal Dopaminergic Neurons
- Efficient Induction of Neural Precursor Cells from Fibroblasts Using Stromal Cell-Derived Inducing Activity
- Efficient Induction of Dopaminergic Neurons from Embryonic Stem Cells for Application to Parkinson's Disease
- Prospect of cell therapy for Parkinson's disease