Cancer Res Treat.
2015 Jan;47(1):26-33. 10.4143/crt.2013.208.
Prospective Evaluation of the Feasibility of Sentinel Lymph Node Biopsy in Breast Cancer Patients with Negative Axillary Conversion after Neoadjuvant Chemotherapy
- Affiliations
-
- 1Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. gsjjoon@yuhs.ac
- 2Department of Surgery, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- KMID: 2380387
- DOI: http://doi.org/10.4143/crt.2013.208
Abstract
- PURPOSE
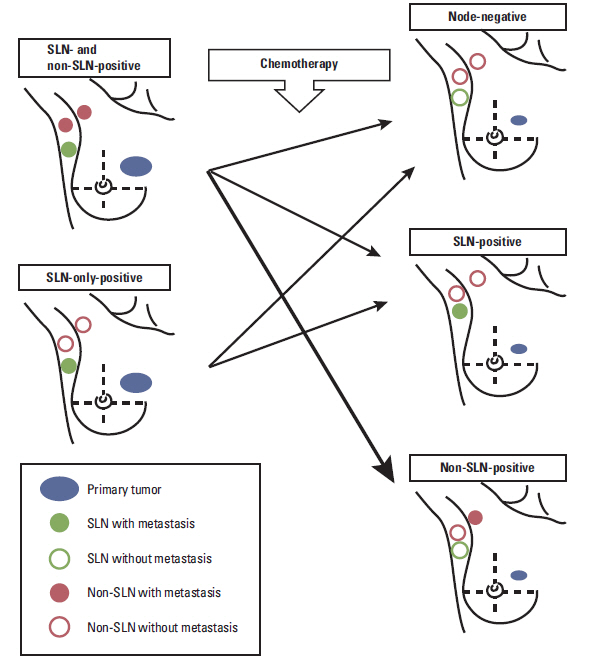
Tumor response to neoadjuvant chemotherapy (NAC) may adversely affect the identification and accuracy rate of sentinel lymph node biopsy (SLNB). This study was conducted to evaluate the feasibility of SLNB in node-positive breast cancer patients with negative axillary conversion after NAC.
MATERIALS AND METHODS
Ninety-six patients with positive nodes at presentation were prospectively enrolled. 18Fluorodeoxyglucose-positron emission tomography (18F-FDG PET) and ultrasonography were performed before and after NAC. A metastatic axillary lymph node was defined as positive if it was positive upon both 18F-FDG PET and ultrasonography, while it was considered negative if it was negative upon both 18F-FDG PET and ultrasonography.
RESULTS
After NAC, 55 cases (57.3%) became clinically node-negative, while 41 cases (42.7%) remained node-positive. In the entire cohort, the sentinel lymph node (SLN) identification and false-negative rates were 84.3% (81/96) and 18.4% (9/49), respectively. In the negative axillary conversion group, the results of SLNB showed an 85.7% (48/55) identification rate and 16.7% (4/24) false-negative rate.
CONCLUSION
For breast cancer patients with clinically positive nodes at presentation, it is difficult to conclude whether the SLN accurately represents the metastatic status of all axillary lymph nodes, even after clinically negative node conversion following NAC.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994; 220:391–8.
Article2. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007; 8:881–8.
Article3. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005; 23:7703–20.
Article4. Schwartz GF, Giuliano AE, Veronesi U; Consensus Conference Committee. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19-22, 2001, Philadelphia, Pennsylvania. Cancer. 2002; 94:2542–51.
Article5. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998; 16:2672–85.
Article6. Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006; 93:539–46.
Article7. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–85.
Article8. Mohsin SK, Weiss HL, Gutierrez MC, Chamness GC, Schiff R, Digiovanna MP, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005; 23:2460–8.
Article9. Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005; 23:2694–702.
Article10. Drew PJ, Kerin MJ, Mahapatra T, Malone C, Monson JR, Turnbull LW, et al. Evaluation of response to neoadjuvant chemoradiotherapy for locally advanced breast cancer with dynamic contrast-enhanced MRI of the breast. Eur J Surg Oncol. 2001; 27:617–20.
Article11. Buchholz TA, Lehman CD, Harris JR, Pockaj BA, Khouri N, Hylton NF, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008; 26:791–7.
Article12. Kinoshita T. Sentinel lymph node biopsy is feasible for breast cancer patients after neoadjuvant chemotherapy. Breast Cancer. 2007; 14:10–5.
Article13. Shen J, Gilcrease MZ, Babiera GV, Ross MI, Meric-Bernstam F, Feig BW, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007; 109:1255–63.
Article14. Lee S, Kim EY, Kang SH, Kim SW, Kim SK, Kang KW, et al. Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative chemotherapy in axillary node-positive breast cancer patients. Breast Cancer Res Treat. 2007; 102:283–8.
Article15. Newman EA, Sabel MS, Nees AV, Schott A, Diehl KM, Cimmino VM, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol. 2007; 14:2946–52.
Article16. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–61.17. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013; 14:609–18.
Article18. Ahn JH, Son EJ, Kim JA, Youk JH, Kim EK, Kwak JY, et al. The role of ultrasonography and FDG-PET in axillary lymph node staging of breast cancer. Acta Radiol. 2010; 51:859–65.
Article19. Giuliano AE, Hawes D, Ballman KV, Whitworth PW, Blumencranz PW, Reintgen DS, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011; 306:385–93.
Article20. National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Breast cancer guidelines, v.2.2012 [Internet]. Fort Washington: National Comprehensive Cancer Network;2012. [cited 2012 Dec 21]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.21. Gil-Rendo A, Zornoza G, Garcia-Velloso MJ, Regueira FM, Beorlegui C, Cervera M, et al. Fluorodeoxyglucose positron emission tomography with sentinel lymph node biopsy for evaluation of axillary involvement in breast cancer. Br J Surg. 2006; 93:707–12.
Article22. Cox CE, Pendas S, Cox JM, Joseph E, Shons AR, Yeatman T, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998; 227:645–51.
Article23. Cortazar P, Zhang L, Untch M, Mehta K, Costantino J, Wolmark N, et al. Meta-analysis results from the collaborative trials in neoadjuvant breast cancer (CTNeoBC). Cancer Res. 2012; 72(24 Suppl 3):S1–11.24. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–804.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Role of Axillary Lymph Node Fine-Needle Aspiration Cytology in Early Breast Cancer With or Without Neoadjuvant Chemotherapy
- Neoadjuvant Chemotherapy Decreases the Identification Rate of Sentinel Lymph Node Biopsy
- Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update
- The Number of Removed Lymph Nodes for an Acceptable False Negative Rate in Sentinel Lymph Node Biopsy for Breast Cancer
- Sentinel Lymph Node Biopsy in Patients with Clinically Negative Lymph Node After Neoadjuvant Chemotherapy