Real-life Efficacy of Omalizumab After 9 Years of Follow-up
- Affiliations
-
- 1Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, Arcispedale Santa Maria Nuova-IRCCS, Reggio Emilia, Italy. menzella.francesco@asmn.re.it
- 2Scientific Directorate, Arcispedale Santa Maria Nuova-IRCCS, Reggio Emilia, Italy.
- KMID: 2378200
- DOI: http://doi.org/10.4168/aair.2017.9.4.368
Abstract
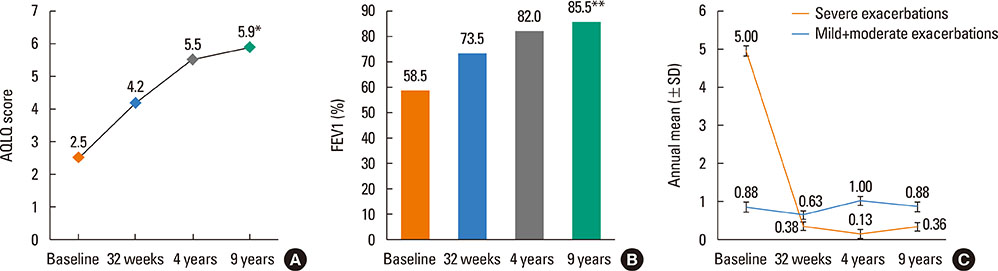
- Omalizumab is frequently used as add-on treatment to inhaled corticosteroids (ICS) and long-acting β2-agonists in patients with suboptimal control of severe asthma. Patients with severe asthma will typically require chronic treatment, although due to the limited amount of data available there are still some concerns about the safety and efficacy of long-term therapy with omalizumab. Herein, in an extension of a previous 4-year study, we report disease-related outcomes of 8 patients with severe persistent allergic asthma who have been followed for a total of 9 years in a real-life setting. Both quality of life (QoL) (evaluated using the Juniper Asthma-Related QoL Questionnaire [AQLQ]) and forced expiratory volume in 1 second (FEV1) showed sustained improvement at 9 years. The median values of AQLQ and FEV1 at 4 years were 5.5 and 82.0% compared to 5.9 and 85.5%, respectively, at 9 years, which were all significantly increased from baseline. After 9 years, the mean annual number of severe exacerbations was 0.63 compared to 5 at baseline. There also appeared to be a trend toward use of a lower dose of ICS at longer follow-up times. After 9 years, there were no safety concerns for continued use of omalizumab, and no asthma-related hospitalizations or emergency department visits were documented over the last 5 years. The present analysis is the longest reported clinical follow-up of omalizumab. Long-term maintenance treatment with omalizumab for up to 9 years is associated with continued benefits in reducing symptoms, exacerbations, and medication burden without any safety concerns.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Characteristics of Adult Severe Refractory Asthma in Korea Analyzed From the Severe Asthma Registry
Min-Hye Kim, Sang-Heon Kim, So-Young Park, Ga-Young Ban, Joo-Hee Kim, Jae-Woo Jung, Ji Yong Moon, Woo-Jung Song, Hyouk-Soo Kwon, Jae-Woo Kwon, Jae Hyun Lee, Hye-Ryun Kang, Jong-Sook Park, Tae-Bum Kim, Heung-Woo Park, Kwang-Ha Yoo, Yeon-Mok Oh, Young-Il Koh, An-Soo Jang, Byung-Jae Lee, Young-Joo Cho, Sang-Heon Cho, Hae-Sim Park, Choon-Sik Park, Ho Joo Yoon, You Sook Cho
Allergy Asthma Immunol Res. 2019;11(1):43-54. doi: 10.4168/aair.2019.11.1.43.Perceptions of Severe Asthma and Asthma-COPD Overlap Syndrome Among Specialists: A Questionnaire Survey
Sang-Heon Kim, Ji Yong Moon, Jae Hyun Lee, Ga-Young Ban, Sujeong Kim, Mi-Ae Kim, Joo-Hee Kim, Min-Hye Kim, Chan-Sun Park, So-Young Park, Hyouk-Soo Kwon, Jae-Woo Kwon, Jae-Woo Jung, Hye-Ryun Kang, Jong-Sook Park, Tae-Bum Kim, Heung-Woo Park, You Sook Cho, Kwang-Ha Yoo, Yeon-Mok Oh, Byung-Jae Lee, An-Soo Jang, Sang-Heon Cho, Hae-Sim Park, Choon-Sik Park, Ho Joo Yoon,
Allergy Asthma Immunol Res. 2018;10(3):225-235. doi: 10.4168/aair.2018.10.3.225.
Reference
-
1. Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996; 9:636–642.2. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31:143–178.3. Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004; 170:836–844.4. Cazzoletti L, Marcon A, Janson C, Corsico A, Jarvis D, Pin I, et al. Asthma control in Europe: a real-world evaluation based on an international population-based study. J Allergy Clin Immunol. 2007; 120:1360–1367.5. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14.6. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014; CD003559.7. Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005; 60:309–316.8. Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011; 139:28–35.9. Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. ‘Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016; 71:593–610.10. Lai T, Wang S, Xu Z, Zhang C, Zhao Y, Hu Y, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015; 5:8191.11. Tzortzaki EG, Georgiou A, Kampas D, Lemessios M, Markatos M, Adamidi T, et al. Long-term omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean “real-life” experience. Pulm Pharmacol Ther. 2012; 25:77–82.12. Zazzali JL, Raimundo KP, Trzaskoma B, Rosén KE, Schatz M. Changes in asthma control, work productivity, and impairment with omalizumab: 5-year EXCELS study results. Allergy Asthma Proc. 2015; 36:283–292.13. Pace E, Ferraro M, Bruno A, Chiappara G, Bousquet J, Gjomarkaj M. Clinical benefits of 7 years of treatment with omalizumab in severe uncontrolled asthmatics. J Asthma. 2011; 48:387–392.14. Menzella F, Facciolongo N, Piro R, Formisano D, Roggeri A, Simonazzi A, et al. Clinical and pharmacoeconomic aspects of omalizumab: a 4-year follow-up. Ther Adv Respir Dis. 2012; 6:87–95.15. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: NIH publication No. 02-3659. Bethesda (MD): National Institutes of Health, National Heart, Lung, and Blood Institute;2002.16. Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis. 1993; 147:832–838.17. Holohan J, Manning P, Nolan D, et al. Asthma control in general practice: adapted from the GINA global strategy for asthma management and prevention. 2nd ed [Internet]. Dublin: Irish College of General Practitioners;2013. 2016 Nov 30. Available from: http://www.hse.ie/eng/about/Who/clinical/natclinprog/asthma/workstreams/asthmacontrol.pdf.18. Li J, Kang J, Wang C, Yang J, Wang L, Kottakis I, et al. Omalizumab improves quality of life and asthma control in Chinese patients with moderate to severe asthma: a randomized phase III study. Allergy Asthma Immunol Res. 2016; 8:319–328.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination of omalizumab and bee venom immunotherapy: does it work?
- Omalizumab on Chronic Spontaneous Urticaria and Chronic Inducible Urticaria: A Real-World Study of Efficacy and Predictors of Treatment Outcome
- Is Omalizumab a Problem-Solving Remedy in Severe Asthma?
- Updated treatment guideline of chronic spontaneous urticaria
- Omalizumab in recurring larynx angioedema: a case report