J Periodontal Implant Sci.
2017 Apr;47(2):86-95. 10.5051/jpis.2017.47.2.86.
Sinus augmentation using rhBMP-2-loaded synthetic bone substitute with simultaneous implant placement in rabbits
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. drjew@yuhs.ac
- 2Department of Periodontology, Kyung Hee University School of Dentistry, Seoul, Korea.
- KMID: 2377743
- DOI: http://doi.org/10.5051/jpis.2017.47.2.86
Abstract
- PURPOSE
The aim of this study was to determine the effect of recombinant human bone morphogenetic protein-2 (rhBMP-2)-loaded synthetic bone substitute on implants that were simultaneously placed with sinus augmentation in rabbits.
METHODS
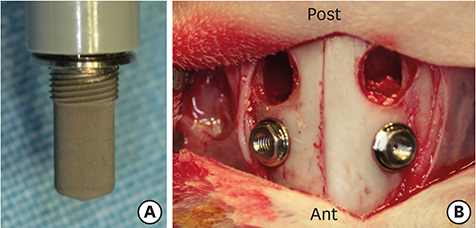
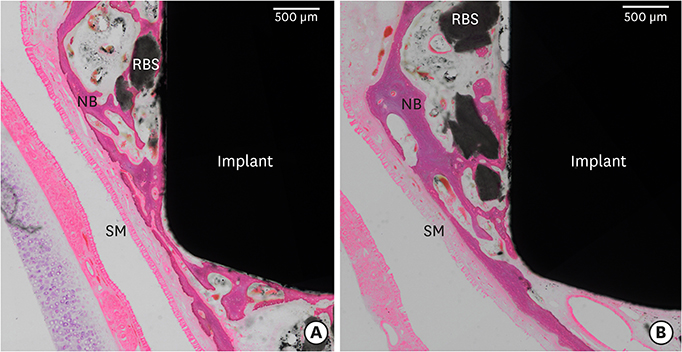
In this study, a circular access window was prepared in the maxillary sinus of rabbits (n=5) for a bone graft around an implant (Ø 3×6 mm) that was simultaneously placed anterior to the window. Synthetic bone substitute loaded with rhBMP-2 was placed on one side of the sinus to form the experimental group, and saline-soaked synthetic bone substitute was placed on the other side of the sinus to form the control group. After 4 weeks, sections were obtained for analysis by micro-computed tomography and histology.
RESULTS
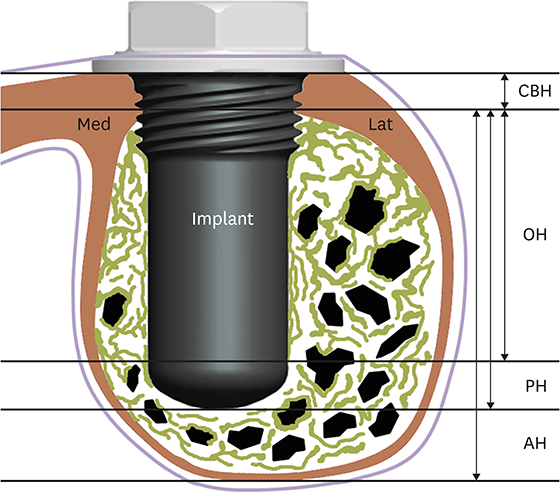
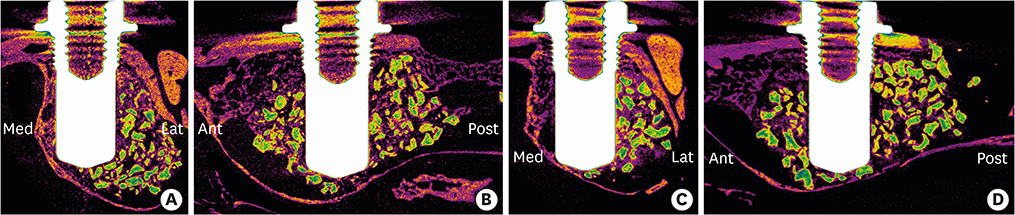
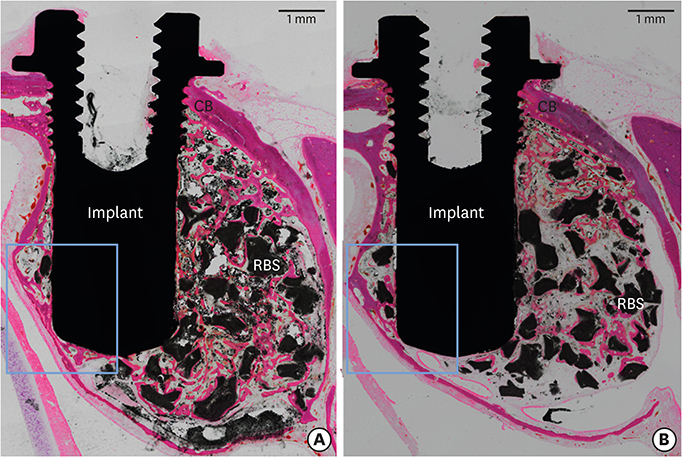
Volumetric analysis showed that the median amount of newly formed bone was significantly greater in the BMP group than in the control group (51.6 mm3 and 46.6 mm3, respectively; P=0.019). In the histometric analysis, the osseointegration height was also significantly greater in the BMP group at the medial surface of the implant (5.2 mm and 4.3 mm, respectively; P=0.037).
CONCLUSIONS
In conclusion, an implant simultaneously placed with sinus augmentation using rhBMP-2-loaded synthetic bone substitute can be successfully osseointegrated, even when only a limited bone height is available during the early stage of healing.
Keyword
MeSH Terms
Figure
Reference
-
1. Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants. 1998; 13:Suppl. 11–45.2. Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003; 8:328–343.
Article3. Peleg M, Mazor Z, Garg AK. Augmentation grafting of the maxillary sinus and simultaneous implant placement in patients with 3 to 5 mm of residual alveolar bone height. Int J Oral Maxillofac Implants. 1999; 14:549–556.4. Peleg M, Mazor Z, Chaushu G, Garg AK. Sinus floor augmentation with simultaneous implant placement in the severely atrophic maxilla. J Periodontol. 1998; 69:1397–1403.
Article5. Felice P, Pistilli R, Piattelli M, Soardi E, Barausse C, Esposito M. 1-stage versus 2-stage lateral sinus lift procedures: 1-year post-loading results of a multicentre randomised controlled trial. Eur J Oral Implantology. 2014; 7:65–75.6. Zitzmann NU, Schärer P. Sinus elevation procedures in the resorbed posterior maxilla. Comparison of the crestal and lateral approaches. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:8–17.7. Jung UW, Hong JY, Lee JS, Kim CS, Cho KS, Choi SH. A hybrid technique for sinus floor elevation in the severely resorbed posterior maxilla. J Periodontal Implant Sci. 2010; 40:76–85.
Article8. Felice P, Scarano A, Pistilli R, Checchi L, Piattelli M, Pellegrino G, et al. A comparison of two techniques to augment maxillary sinuses using the lateral window approach: rigid synthetic resorbable barriers versus anorganic bovine bone. Five-month post-loading clinical and histological results of a pilot randomised controlled clinical trial. Eur J Oral Implantology. 2009; 2:293–306.9. Choi Y, Lee JS, Kim YJ, Kim MS, Choi SH, Cho KS, et al. Recombinant human bone morphogenetic protein-2 stimulates the osteogenic potential of the Schneiderian membrane: a histometric analysis in rabbits. Tissue Eng Part A. 2013; 19:1994–2004.
Article10. Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine. 2002; 27:S16–S23.
Article11. Jung IH, Lim HC, Lee EU, Lee JS, Jung UW, Choi SH. Comparative analysis of carrier systems for delivering bone morphogenetic proteins. J Periodontal Implant Sci. 2015; 45:136–144.
Article12. Alam I, Asahina I, Ohmamiuda K, Enomoto S. Comparative study of biphasic calcium phosphate ceramics impregnated with rhBMP-2 as bone substitutes. J Biomed Mater Res. 2001; 54:129–138.
Article13. Brodie JC, Goldie E, Connel G, Merry J, Grant MH. Osteoblast interactions with calcium phosphate ceramics modified by coating with type I collagen. J Biomed Mater Res A. 2005; 73:409–421.
Article14. Kim JS, Cha JK, Cho AR, Kim MS, Lee JS, Hong JY, et al. Acceleration of bone regeneration by BMP-2-loaded collagenated biphasic calcium phosphate in rabbit sinus. Clin Implant Dent Relat Res. 2015; 17:1103–1113.
Article15. Watanabe K, Niimi A, Ueda M. Autogenous bone grafts in the rabbit maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:26–32.
Article16. Wada K, Niimi A, Watanabe K, Sawai T, Ueda M. Maxillary sinus floor augmentation in rabbits: a comparative histologic-histomorphometric study between rhBMP-2 and autogenous bone. Int J Periodontics Restorative Dent. 2001; 21:252–263.17. Sun XJ, Zhang ZY, Wang SY, Gittens SA, Jiang XQ, Chou LL. Maxillary sinus floor elevation using a tissue-engineered bone complex with OsteoBone and bMSCs in rabbits. Clin Oral Implants Res. 2008; 19:804–813.
Article18. Kim YS, Kim SH, Kim KH, Jhin MJ, Kim WK, Lee YK, et al. Rabbit maxillary sinus augmentation model with simultaneous implant placement: differential responses to the graft materials. J Periodontal Implant Sci. 2012; 42:204–211.
Article19. Brunner E, Langer F. Nonparametric analysis of ordered categorical data in designs with longitudinal observations and small sample sizes. Biom J. 2000; 42:663–675.
Article20. Wikesjö UM, Qahash M, Thomson RC, Cook AD, Rohrer MD, Wozney JM, et al. rhBMP-2 significantly enhances guided bone regeneration. Clin Oral Implants Res. 2004; 15:194–204.
Article21. Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992; 74:659–670.
Article22. Sigurdsson TJ, Fu E, Tatakis DN, Rohrer MD, Wikesjö UM. Bone morphogenetic protein-2 for peri-implant bone regeneration and osseointegration. Clin Oral Implants Res. 1997; 8:367–374.
Article23. Guillot R, Gilde F, Becquart P, Sailhan F, Lapeyrere A, Logeart-Avramoglou D, et al. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials. 2013; 34:5737–5746.
Article24. Jung UW, Unursaikhan O, Park JY, Lee JS, Otgonbold J, Choi SH. Tenting effect of the elevated sinus membrane over an implant with adjunctive use of a hydroxyapatite-powdered collagen membrane in rabbits. Clin Oral Implants Res. 2015; 26:663–670.
Article25. Chen D, Zhao M, Harris SE, Mi Z. Signal transduction and biological functions of bone morphogenetic proteins. Front Biosci. 2004; 9:349–358.
Article26. Khoury F. Augmentation of the sinus floor with mandibular bone block and simultaneous implantation: a 6-year clinical investigation. Int J Oral Maxillofac Implants. 1999; 14:557–564.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Delayed Occurrence of Maxillary Sinusitis after Simultaneous Maxillary Sinus Augmentation and Implant: A Case Report and Literature Review
- Rabbit maxillary sinus augmentation model with simultaneous implant placement: differential responses to the graft materials
- Bone formation around rhBMP-2-coated implants in rabbit sinuses with or without absorbable collagen sponge grafting
- Sinus floor augmentation using a mineralized freeze-dried bone graft combined with recombinant human bone morphogenetic protein-2: clinical and histological results of three cases
- Effect of rhPMP-2 coated implants on alveolar ridge augmentation in dogs