J Periodontal Implant Sci.
2012 Dec;42(6):204-211.
Rabbit maxillary sinus augmentation model with simultaneous implant placement: differential responses to the graft materials
- Affiliations
-
- 1Department of Periodontics, Asan Medical Center, Seoul, Korea.
- 2Department of Dentistry, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. ymlee@snu.ac.kr
Abstract
- PURPOSE
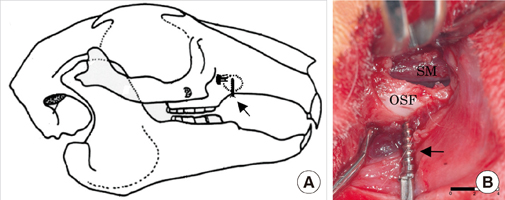
This study was performed to establish an experimental rabbit model for single-stage maxillary sinus augmentation with simultaneous implant placement.
METHODS
Twelve mature New Zealand white rabbits were used for the experiments. The rabbit maxillary sinuses were divided into 3 groups according to sinus augmentation materials: blood clot (BC), autogenous bone (AB), and bovine-derived hydroxyapatite (BHA). Small titanium implants were simultaneously placed in the animals during the sinus augmentation procedure. The rabbits were sacrificed 4 and 8 weeks after surgery and were observed histologically. Histomorphometric analyses using image analysis software were also performed to evaluate the parameters related to bone regeneration and implant-bone integration.
RESULTS
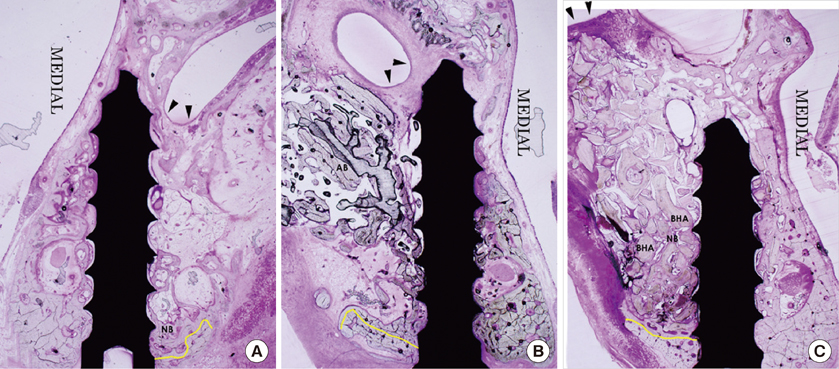
The BC group showed an evident collapse of the sinus membrane and limited new bone formation around the original sinus floor at 4 and 8 weeks. In the AB group, the sinus membrane was well retained above the implant apex, and new bone formation was significant at both examination periods. The BHA group also showed retention of the elevated sinus membrane above the screw apex and evident new bone formation at both points in time. The total area of the mineral component (TMA) in the area of interest and the bone-to-implant contact did not show any significant differences among all the groups. In the AB group, the TMA had significantly decreased from 4 to 8 weeks.
CONCLUSIONS
Within the limits of this study, the rabbit sinus model showed satisfactory results in the comparison of different grafting conditions in single-stage sinus floor elevation with simultaneous implant placement. We found that the rabbit model was useful for maxillary sinus augmentation with simultaneous implant placement.
Keyword
MeSH Terms
-
Animals
Bone Regeneration
Bone Substitutes
Butylated Hydroxyanisole
Dental Implants
Durapatite
Floors and Floorcoverings
Guided Tissue Regeneration
Maxillary Sinus
Membranes
Models, Animal
Osteogenesis
Rabbits
Retention (Psychology)
Sinus Floor Augmentation
Titanium
Transplants
Bone Substitutes
Butylated Hydroxyanisole
Dental Implants
Durapatite
Titanium
Figure
Reference
-
1. Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986. 30:207–229.
Article2. Summers RB. A new concept in maxillary implant surgery: the osteotome technique. Compendium. 1994. 15:152154–156. 158
Article3. Rosen PS, Summers R, Mellado JR, Salkin LM, Shanaman RH, Marks MH, et al. The bone-added osteotome sinus floor elevation technique: multicenter retrospective report of consecutively treated patients. Int J Oral Maxillofac Implants. 1999. 14:853–858.
Article4. Fugazzotto PA. The modified trephine/osteotome sinus augmentation technique: technical considerations and discussion of indications. Implant Dent. 2001. 10:259–264.
Article5. Chen L, Cha J. An 8-year retrospective study: 1,100 patients receiving 1,557 implants using the minimally invasive hydraulic sinus condensing technique. J Periodontol. 2005. 76:482–491.6. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980. 38:613–616.7. Lazzara RJ. The sinus elevation procedure in endosseous implant therapy. Curr Opin Periodontol. 1996. 3:178–183.
Article8. Peleg M, Mazor Z, Chaushu G, Garg AK. Sinus floor augmentation with simultaneous implant placement in the severely atrophic maxilla. J Periodontol. 1998. 69:1397–1403.
Article9. Zitzmann NU, Scharer P. Sinus elevation procedures in the resorbed posterior maxilla. Comparison of the crestal and lateral approaches. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:8–17.10. Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants. 1998. 13:Suppl. 11–45.11. Peleg M, Mazor Z, Garg AK. Augmentation grafting of the maxillary sinus and simultaneous implant placement in patients with 3 to 5 mm of residual alveolar bone height. Int J Oral Maxillofac Implants. 1999. 14:549–556.
Article12. Mayfield LJ, Skoglund A, Hising P, Lang NP, Attstrom R. Evaluation following functional loading of titanium fixtures placed in ridges augmented by deproteinized bone mineral. A human case study. Clin Oral Implants Res. 2001. 12:508–514.
Article13. Fugazzotto PA. Immediate implant placement following a modified trephine/osteotome approach: success rates of 116 implants to 4 years in function. Int J Oral Maxillofac Implants. 2002. 17:113–120.
Article14. Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003. 8:328–343.
Article15. Emmerich D, Att W, Stappert C. Sinus floor elevation using osteotomes: a systematic review and meta-analysis. J Periodontol. 2005. 76:1237–1251.
Article16. Ferrigno N, Laureti M, Fanali S. Dental implants placement in conjunction with osteotome sinus floor elevation: a 12-year life-table analysis from a prospective study on 588 ITI implants. Clin Oral Implants Res. 2006. 17:194–205.
Article17. Watanabe K, Niimi A, Ueda M. Autogenous bone grafts in the rabbit maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999. 88:26–32.18. Wada K, Niimi A, Watanabe K, Sawai T, Ueda M. Maxillary sinus floor augmentation in rabbits: a comparative histologic-histomorphometric study between rhBMP-2 and autogenous bone. Int J Periodontics Restorative Dent. 2001. 21:252–263.19. Butterfield KJ, Bennett J, Gronowicz G, Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005. 63:370–376.20. Ohya M, Yamada Y, Ozawa R, Ito K, Takahashi M, Ueda M. Sinus floor elevation applied tissue-engineered bone. Comparative study between mesenchymal stem cells/platelet-rich plasma (PRP) and autogenous bone with PRP complexes in rabbits. Clin Oral Implants Res. 2005. 16:622–629.21. Rahmani M, Shimada E, Rokni S, Deporter DA, Adegbembo AO, Valiquette N, et al. Osteotome sinus elevation and simultaneous placement of porous-surfaced dental implants: a morphometric study in rabbits. Clin Oral Implants Res. 2005. 16:692–699.22. Sun XJ, Zhang ZY, Wang SY, Gittens SA, Jiang XQ, Chou LL. Maxillary sinus floor elevation using a tissue-engineered bone complex with OsteoBone and bMSCs in rabbits. Clin Oral Implants Res. 2008. 19:804–813.23. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J Oral Pathol. 1982. 11:318–326.
Article24. Dannan A, Alkattan F. Animal models in periodontal research: a mini-review of the literature. Internet J Vet Med [Internet]. 2008. cited 2009 Aug 12. 5:about 3. Available from: http://www.ispub.com/journal/the-internet-journal-of-veterinary-medicine/volume-5-number-1/animal-models-in-periodontal-research-a-mini-review-of-the-literature.html#sthash.Jpt0qK5R.dpbs.
Article25. Schlegel AK, Donath K. BIO-OSS: a resorbable bone substitute? J Long Term Eff Med Implants. 1998. 8:201–209.26. McAllister BS, Margolin MD, Cogan AG, Buck D, Hollinger JO, Lynch SE. Eighteen-month radiographic and histologic evaluation of sinus grafting with anorganic bovine bone in the chimpanzee. Int J Oral Maxillofac Implants. 1999. 14:361–368.27. Handschel J, Simonowska M, Naujoks C, Depprich RA, Ommerborn MA, Meyer U, et al. A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 2009. 5:12.
Article28. Lee YM, Shin SY, Kim JY, Kye SB, Ku Y, Rhyu IC. Bone reaction to bovine hydroxyapatite for maxillary sinus floor augmentation: histologic results in humans. Int J Periodontics Restorative Dent. 2006. 26:471–481.
Article29. Hallman M, Cederlund A, Lindskog S, Lundgren S, Sennerby L. A clinical histologic study of bovine hydroxyapatite in combination with autogenous bone and fibrin glue for maxillary sinus floor augmentation. Results after 6 to 8 months of healing. Clin Oral Implants Res. 2001. 12:135–143.
Article30. Johansson CB, Albrektsson T. A removal torque and histomorphometric study of commercially pure niobium and titanium implants in rabbit bone. Clin Oral Implants Res. 1991. 2:24–29.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Delayed Occurrence of Maxillary Sinusitis after Simultaneous Maxillary Sinus Augmentation and Implant: A Case Report and Literature Review
- Vertical Augmentation of Maxillary Posterior Alveolar Ridge Using Allogenic Block Bone Graft and Simultaneous Maxillary Sinus Graft
- Resorption of bone graft after maxillary sinus grafting and simultaneous implant placement
- One-staged sinus bone graft using tapered porous surfaced implant in lower residual bone height of maxillary sinus
- Maxillary sinus bone graft using particulated ramal autobone and bovine bone