Korean Circ J.
2017 Mar;47(2):160-167. 10.4070/kcj.2016.0280.
Spatial Allocation and Specification of Cardiomyocytes during Zebrafish Embryogenesis
- Affiliations
-
- 1Department of Cell Biology, National Cerebral and Cardiovascular Center Research Institute, Osaka, Japan. nmochizu@ri.ncvc.go.jp
- 2Management office, National Center for Child Health and Development, Tokyo, Japan.
- 3AMED-CREST, National Cerebral and Cardiovascular Center Research Institute, Osaka, Japan.
- KMID: 2377452
- DOI: http://doi.org/10.4070/kcj.2016.0280
Abstract
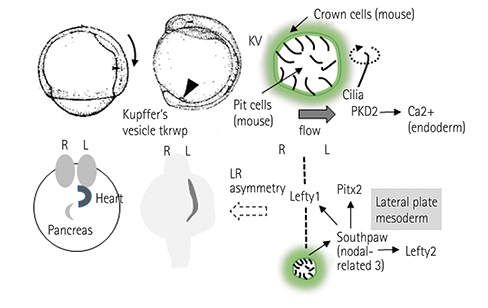
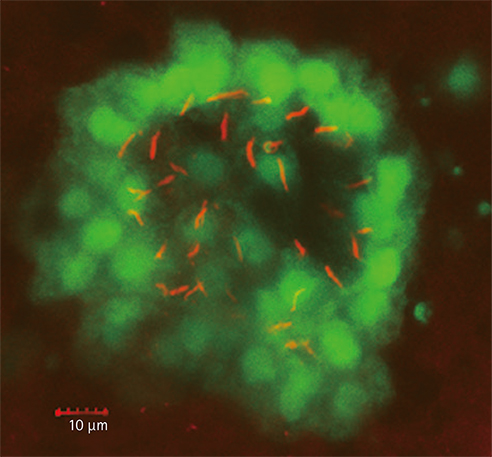
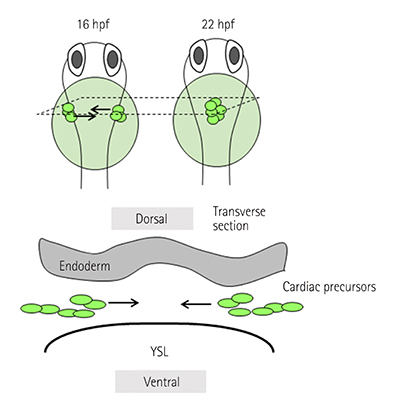
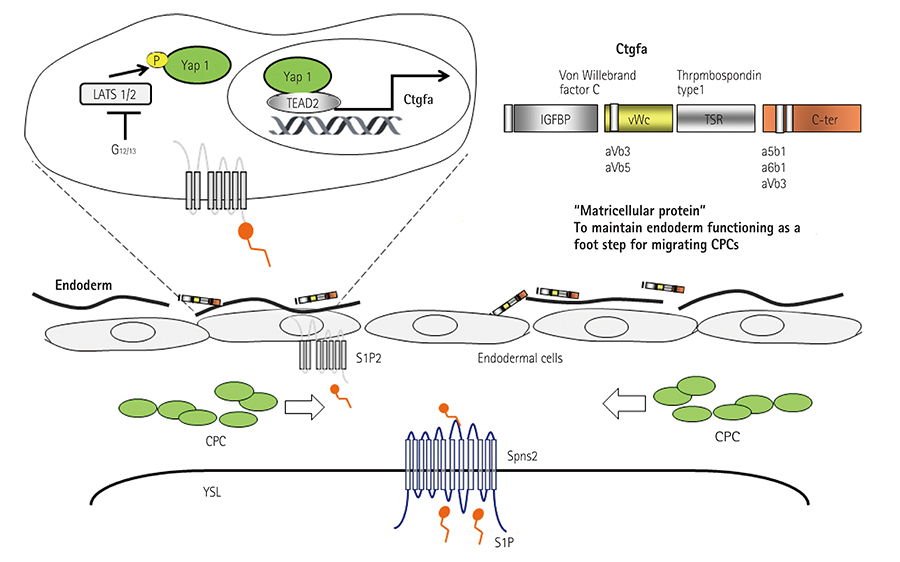
- Incomplete development and severe malformation of the heart result in miscarriage of embryos because of its malfunction as a pump for circulation. During cardiogenesis, development of the heart is precisely coordinated by the genetically-primed program that is revealed by the sequential expression of transcription factors. It is important to investigate how spatial allocation of the heart containing cardiomyocytes and other mesoderm-derived cells is determined. In addition, the molecular mechanism underlying cardiomyocyte differentiation still remains elusive. The location of ectoderm-, mesoderm-, and endoderm-derived organs is determined by their initial allocation and subsequent mutual cell-cell interactions or paracrine-based regulation. In the present work, we provide an overview of cardiac development controlled by the germ layers and discuss the points that should be uncovered in future for understanding cardiogenesis.
MeSH Terms
Figure
Cited by 1 articles
-
Prolonged Intensive Exercise: When the Right Ventricle Goes Wrong
Yoonjee Park, Eung Ju Kim
Korean Circ J. 2018;48(11):1025-1027. doi: 10.4070/kcj.2018.0218.
Reference
-
1. Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000; 14:839–854.2. Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988; 319:189–194.3. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002; 39:1890–1900.4. Moller JH, Taubert KA, Allen HD, Clark EB, Lauer RM. Cardiovascular health and disease in children: current status. A Special Writing Group from the Task Force on Children and Youth, American Heart Association. Circulation. 1994; 89:923–930.5. Tong YF. Mutations of NKX2.5 and GATA4 genes in the development of congenital heart disease. Gene. 2016; 588:86–94.6. McCulley DJ, Black BL. Transcription factor pathways and congenital heart disease. Curr Top Dev Biol. 2012; 100:253–277.7. Ramsdell AF. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev Biol. 2005; 288:1–20.8. Patton EE, Zon LI. The art and design of genetic screens: zebrafish. Nat Rev Genet. 2001; 2:956–966.9. Santoriello C, Zon LI. Hooked! Modeling human disease in zebrafish. J Clin Invest. 2012; 122:2337–2343.10. Auer TO, Del BF. CRISPR/Cas9 and TALEN-mediated knock-in approaches in zebrafish. Methods. 2014; 69:142–150.11. Driever W, Fishman MC. The zebrafish: heritable disorders in transparent embryos. J Clin Invest. 1996; 97:1788–1794.12. Chen JN, Fishman MC. Genetics of heart development. Trends Genet. 2000; 16:383–388.13. Yelon D. Cardiac patterning and morphogenesis in zebrafish. Dev Dyn. 2001; 222:552–563.14. Rottbauer W, Wessels G, Dahme T, et al. Cardiac myosin light chain-2: a novel essential component of thick-myofilament assembly and contractility of the heart. Circ Res. 2006; 99:323–331.15. Guner-Ataman B, Paffett-Lugassy N, Adams MS, et al. Zebrafish second heart field development relies on progenitor specification in anterior lateral plate mesoderm and nkx2.5 function. Development. 2013; 140:1353–1363.16. Lazic S, Scott IC. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev Biol. 2011; 354:123–133.17. Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005; 39:561–613.18. D'Amico LA, Cooper MS. Spatially distinct domains of cell behavior in the zebrafish organizer region. Biochem Cell Biol. 1997; 75:563–577.19. Matsui T, Ishikawa H, Bessho Y. Cell collectivity regulation within migrating cell cluster during Kupffer's vesicle formation in zebrafish. Front Cell Dev Biol. 2015; 3:27.20. Okabe N, Xu B, Burdine RD. Fluid dynamics in zebrafish Kupffer's vesicle. Dev Dyn. 2008; 237:3602–3612.21. Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005; 132:1907–1921.22. Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003; 130:2303–2316.23. Keegan BR, Meyer D, Yelon D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development. 2004; 131:3081–3091.24. Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D. Retinoic acid signaling restricts the cardiac progenitor pool. Science. 2005; 307:247–249.25. Ueno S, Weidinger G, Osugi T, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007; 104:9685–9690.26. Marques SR, Lee Y, Poss KD, Yelon D. Reiterative roles for FGF signaling in the establishment of size and proportion of the zebrafish heart. Dev Biol. 2008; 321:397–406.27. Marques SR, Yelon D. Differential requirement for BMP signaling in atrial and ventricular lineages establishes cardiac chamber proportionality. Dev Biol. 2009; 328:472–482.28. Reiter JF, Verkade H, Stainier DY. Bmp2b and Oep promote early myocardial differentiation through their regulation of gata5. Dev Biol. 2001; 234:330–338.29. Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell. 2007; 12:391–402.30. Reiter JF, Alexander J, Rodaway A, et al. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999; 13:2983–2995.31. Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001; 128:3081–3094.32. Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005; 132:1247–1260.33. Kikuchi Y, Agathon A, Alexander J, et al. Casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001; 15:1493–1505.34. Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol. 2004; 14:685–690.35. Mizoguchi T, Verkade H, Heath JK, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development. 2008; 135:2521–2529.36. Matsui T, Thitamadee S, Murata T, et al. Canopy1, a positive feedback regulator of FGF signaling, controls progenitor cell clustering during Kupffer's vesicle organogenesis. Proc Natl Acad Sci USA. 2011; 108:9881–9886.37. Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009; 458:651–654.38. Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005; 435:172–177.39. Pennekamp P, Karcher C, Fischer A, et al. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002; 12:938–943.40. Takao D, Nemoto T, Abe T, et al. Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left-right axis formation. Dev Biol. 2013; 376:23–30.41. Sarmah B, Latimer AJ, Appel B, Wente SR. Inositol polyphosphates regulate zebrafish left-right asymmetry. Dev Cell. 2005; 9:133–145.42. Yuan S, Zhao L, Brueckner M, Sun Z. Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr Biol. 2015; 25:556–567.43. Roxo-Rosa M, Jacinto R, Sampaio P, Lopes SS. The zebrafish Kupffer's vesicle as a model system for the molecular mechanisms by which the lack of Polycystin-2 leads to stimulation of CFTR. Biol Open. 2015; 4:1356–1366.44. Lahvic JL, Ji Y, Marin P, et al. Small heat shock proteins are necessary for heart migration and laterality determination in zebrafish. Dev Biol. 2013; 384:166–180.45. Delling M, Indzhykulian AA, Liu X, et al. Primary cilia are not calcium-responsive mechanosensors. Nature. 2016; 531:656–660.46. Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013; 188:913–922.47. Rao DR, Gabriel GC, Li Y, et al. Role of cilia in structural birth defects: insights from ciliopathy mutant mouse models. Birth Defects Res C Embryo Today. 2014; 102:115–125.48. Kennedy MP, Omran H, Leigh MW, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007; 115:2814–2821.49. McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003; 13:385–392.50. Stainier DY, Lee RK, Fishman MC. Cardiovascular development in the zebrafish I Myocardial fate map and heart tube formation. Development. 1993; 119:31–40.51. Dickmeis T, Mourrain P, Saint-Etienne L, et al. A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 2001; 15:1487–1492.52. Sakaguchi T, Kikuchi Y, Kuroiwa A, Takeda H, Stainier DY. The yolk syncytial layer regulates myocardial migration by influencing extracellular matrix assembly in zebrafish. Development. 2006; 133:4063–4072.53. Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000; 406:192–195.54. Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009; 323:524–527.55. Osborne N, Brand-Arzamendi K, Ober EA, et al. The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr Biol. 2008; 18:1882–1888.56. Liu J, Stainier DY. Zebrafish in the study of early cardiac development. Circ Res. 2012; 110:870–874.57. Zhou Y, Cashman TJ, Nevis KR, et al. Latent TGF-beta binding protein 3 identifies a second heart field in zebrafish. Nature. 2011; 474:645–648.58. Fukui H, Terai K, Nakajima H, Chiba A, Fukuhara S, Mochizuki N. S1P-Yap1 signaling regulates endoderm formation required for cardiac precursor cell migration in zebrafish. Dev Cell. 2014; 31:128–136.59. Mendelson K, Evans T, Hla T. Sphingosine 1-phosphate signalling. Development. 2014; 141:5–9.60. Xiang SY, Dusaban SS, Brown JH. Lysophospholipid receptor activation of RhoA and lipid signaling pathways. Biochim Biophys Acta. 2013; 1831:213–222.61. Clay H, Wilsbacher LD, Wilson SJ, et al. Sphingosine 1-phosphate receptor-1 in cardiomyocytes is required for normal cardiac development. Dev Biol. 2016; 418:157–165.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Zebrafish and Mycobacterial infection
- Zebrafish Vascular Development: General and Tissue-Specific Regulation
- p53 Promotes Differentiation of Cardiomyocytes from hiPSC through Wnt Signaling-Mediated Mesendodermal Differentiation
- Possibility of Zebrafish as New infection model for Leprosy
- Zebrafish as a model for human epithelial pathology