J Breast Cancer.
2013 Mar;16(1):32-39.
Tumor-Associated Lymphocytes Predict Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. gygong@amc.seoul.kr
- 2Department of Surgery, Changwon Fatima Hospital, Changwon, Korea.
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Division of Breast and Endocrine Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Tumor-associated lymphocyte numbers in breast cancer have been suggested as a new independent predictor of response to neoadjuvant chemotherapy in breast cancer patients. We therefore evaluated the relationship between pathologic complete response (pCR) and tumor-associated lymphocytes in tumors of such patients.
METHODS
Between 2000 and 2009, we retrospectively evaluated 175 patients with primary breast cancer treated with neoadjuvant chemotherapy, followed by definitive surgical resection. Peritumoral lymphocytic infiltration (LI) and CD3+, CD8+, and forkhead box P3 (FOXP3)+ lymphocytes were assessed in pretreatment biopsy specimens.
RESULTS
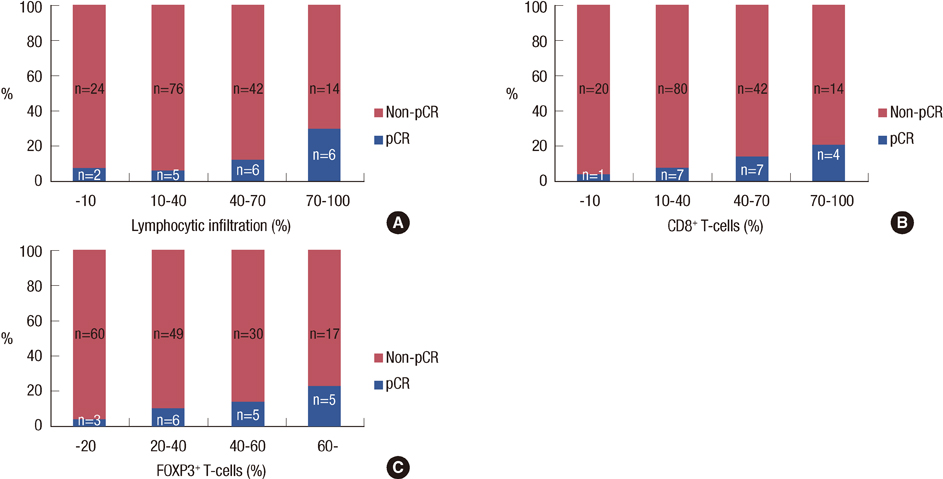
Nineteen (11%) patients achieved pCR. An elevated LI, CD3+, CD8+, or FOXP3+ lymphocytic infiltration; lower clinical T stage; human epidermal growth factor receptor 2 overexpression; and herceptin-based treatment were all significantly associated with pCR. Through a multivariate analysis, LI (odds ratio [OR], 1.26; p=0.024), clinical T stage (OR, 3.06; p=0.041), and the use of a herceptin-based regimen (OR, 4.95; p=0.004) were all significant independent predictors of pCR. Significantly higher numbers of tumor-associated lymphocytes and CD3+, CD8+, and FOXP3+ T-cells were observed in the following: high-grade tumors, tumors of positive nodal status, and tumors negative for hormone receptors.
CONCLUSION
Tumor-associated lymphocytes are significantly associated with pCR, suggesting that tumor-associated lymphocytes may be an important pathological factor predicting a response to neoadjuvant chemotherapy in breast cancer patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003. 21:4165–4174.
Article2. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997. 15:2483–2493.
Article3. Abrial SC, Penault-Llorca F, Delva R, Bougnoux P, Leduc B, Mouret-Reynier MA, et al. High prognostic significance of residual disease after neoadjuvant chemotherapy: a retrospective study in 710 patients with operable breast cancer. Breast Cancer Res Treat. 2005. 94:255–263.
Article4. Tewari M, Krishnamurthy A, Shukla HS. Predictive markers of response to neoadjuvant chemotherapy in breast cancer. Surg Oncol. 2008. 17:301–311.
Article5. Faneyte IF, Schrama JG, Peterse JL, Remijnse PL, Rodenhuis S, van de Vijver MJ. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer. 2003. 88:406–412.
Article6. DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007. 9:212.
Article7. Schmidt M, Böhm D, von Törne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008. 68:5405–5413.
Article8. Tomsová M, Melichar B, Sedláková I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008. 108:415–420.
Article9. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003. 348:203–213.
Article10. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005. 102:18538–18543.
Article11. Menard S, Tomasic G, Casalini P, Balsari A, Pilotti S, Cascinelli N, et al. Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 1997. 3:817–819.12. Ridolfi RL, Rosen PP, Port A, Kinne D, Miké V. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977. 40:1365–1385.
Article13. Kuroda H, Tamaru J, Sakamoto G, Ohnisi K, Itoyama S. Immunophenotype of lymphocytic infiltration in medullary carcinoma of the breast. Virchows Arch. 2005. 446:10–14.
Article14. Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010. 28:105–113.
Article15. Hornychova H, Melichar B, Tomsova M, Mergancova J, Urminska H, Ryska A. Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest. 2008. 26:1024–1031.
Article16. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008. 8:59–73.
Article17. Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008. 14:2413–2420.
Article18. Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006. 24:5373–5380.
Article19. Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999. 17:1983–1987.20. Chollet P, Amat S, Cure H, de Latour M, Le Bouedec G, Mouret-Reynier MA, et al. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002. 86:1041–1046.
Article21. Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008. 57:1579–1587.
Article22. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.
Article23. Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008. 118:1991–2001.
Article24. Soliman H. Developing an effective breast cancer vaccine. Cancer Control. 2010. 17:183–190.
Article25. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009. 30:636–645.
Article26. Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006. 24:209–226.
Article27. Oda N, Shimazu K, Naoi Y, Morimoto K, Shimomura A, Shimoda M, et al. Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Res Treat. 2012. 136:107–116.
Article28. Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods. 2008. 333:167–179.
Article29. Alexe G, Dalgin GS, Scanfeld D, Tamayo P, Mesirov JP, DeLisi C, et al. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res. 2007. 67:10669–10676.
Article30. Hanrahan EO, Hennessy BT, Valero V. Neoadjuvant systemic therapy for breast cancer: an overview and review of recent clinical trials. Expert Opin Pharmacother. 2005. 6:1477–1491.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Tumor-infiltrating Lymphocytes in Breast Cancer after Neoadjuvant Chemotherapy
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Predictive and Prognostic Roles of Pathological Indicators for Patients with Breast Cancer on Neoadjuvant Chemotherapy
- Pathologic Findings of Residual Tumor according to the Response Rate after Neoadjuvant Chemotherapy for Breast Cancer
- RECIST Criteria for Tumor Response in the Patients with Breast Cancer Who Had Neoadjuvant Chemotherapy