Yonsei Med J.
2016 May;57(3):580-587. 10.3349/ymj.2016.57.3.580.
Clinical Significance of CA125 Level after the First Cycle of Chemotherapy on Survival of Patients with Advanced Ovarian Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Yonsei University Graduate School, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jaehoonkim@yuhs.ac
- 4Biostatistics Collaboration Lab, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Institute of Women's Medical Life Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. shkim70@yuhs.ac
- KMID: 2374075
- DOI: http://doi.org/10.3349/ymj.2016.57.3.580
Abstract
- PURPOSE
To determine the most powerful cancer antigen 125 (CA125)-related prognostic factor for advanced epithelial ovarian cancer (EOC) and to identify cut-off values that distinguish patients with a poor prognosis from those with a good prognosis.
MATERIALS AND METHODS
We included 223 patients who received staging laparotomy and were diagnosed with stage IIC-IV serous EOC. Cox regression analysis was used to determine the most significant prognostic factor among the following variables: serum CA125 before surgery and after the first, second, and sixth cycles of chemotherapy; the nadir CA125 value; the relative percentage change in CA125 levels after the first and second cycles of chemotherapy compared to baseline CA125; CA125 half-life; time to nadir; and time to normalization of the CA125 level.
RESULTS
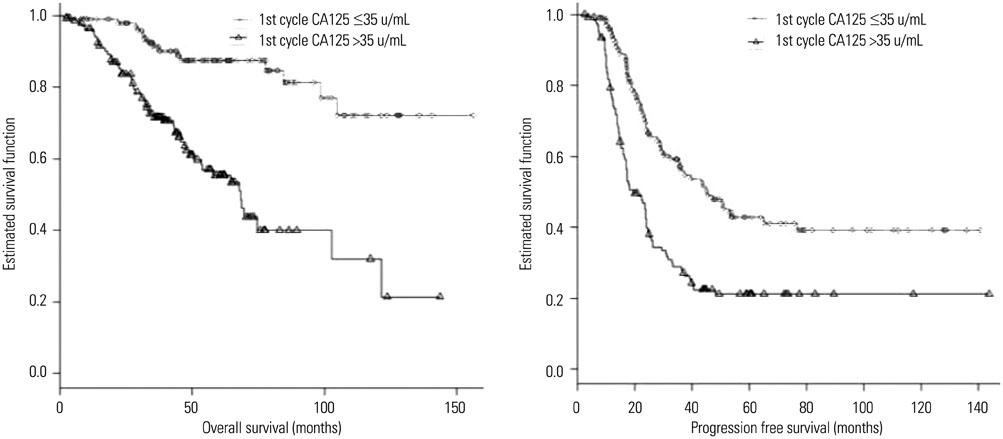
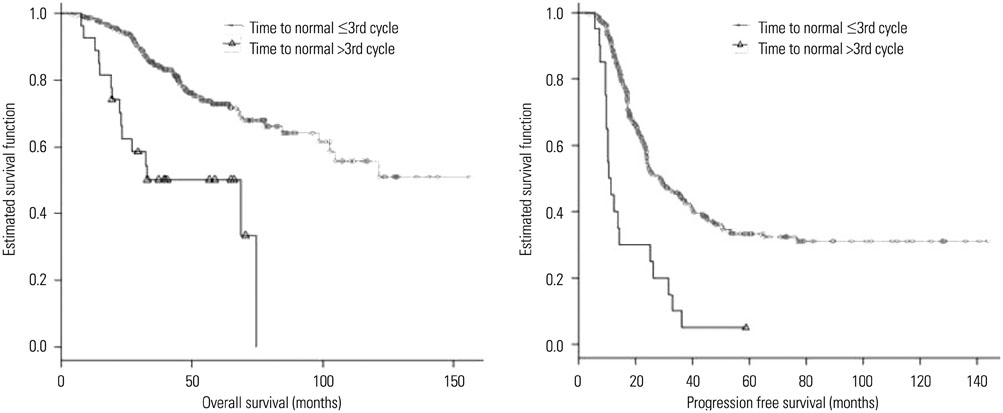
The CA125 level after the first chemotherapy cycle was the most significant independent prognostic factor for overall survival (OS). Time to normalization (p=0.028) and relative percentage change between CA125 levels at baseline and after the first chemotherapy cycle (p=0.021) were additional independent prognostic factors in terms of OS. The CA125 level after the first chemotherapy cycle (p=0.001) and time to normalization (p<0.001) were identified as independent prognostic factors for progression free survival (PFS).
CONCLUSION
Among well-established CA125-related prognostic factors, serum CA125 levels after the first cycle of chemotherapy and time to normalization were the most significant prognostic factors for both OS and PFS.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Antineoplastic Agents/*therapeutic use
CA-125 Antigen/*blood/metabolism
Disease-Free Survival
Female
Humans
Middle Aged
Neoplasm Staging
Neoplasms, Glandular and Epithelial/*blood/*drug therapy/mortality
Ovarian Neoplasms/*blood/*drug therapy/mortality
Prognosis
Regression Analysis
Antineoplastic Agents
CA-125 Antigen
Figure
Reference
-
1. Krivak TC, Tian C, Rose GS, Armstrong DK, Maxwell GL. A Gynecologic Oncology Group study of serum CA-125 levels in patients with stage III optimally debulked ovarian cancer treated with intraperitoneal compared to intravenous chemotherapy: an analysis of patients enrolled in GOG 172. Gynecol Oncol. 2009; 115:81–85.
Article2. Micha JP, Goldstein BH, Rettenmaier MA, Brown JV 3rd, John CR, Markman M. Clinical utility of CA-125 for maintenance therapy in the treatment of advanced stage ovarian carcinoma. Int J Gynecol Cancer. 2009; 19:239–241.
Article3. Richardson DL, Seamon LG, Carlson MJ, O'Malley DM, Fowler JM, Copeland LJ, et al. CA125 decline in ovarian cancer patients treated with intravenous versus intraperitoneal platinum-based chemotherapy. Gynecol Oncol. 2008; 111:233–236.
Article4. Rocconi RP, Matthews KS, Kemper MK, Hoskins KE, Huh WK, Straughn JM Jr. The timing of normalization of CA-125 levels during primary chemotherapy is predictive of survival in patients with epithelial ovarian cancer. Gynecol Oncol. 2009; 114:242–245.
Article5. Tian C, Markman M, Zaino R, Ozols RF, McGuire WP, Muggia FM, et al. CA-125 change after chemotherapy in prediction of treatment outcome among advanced mucinous and clear cell epithelial ovarian cancers: a Gynecologic Oncology Group study. Cancer. 2009; 115:1395–1403.
Article6. Prat A, Parera M, Peralta S, Perez-Benavente MA, Garcia A, Gil-Moreno A, et al. Nadir CA-125 concentration in the normal range as an independent prognostic factor for optimally treated advanced epithelial ovarian cancer. Ann Oncol. 2008; 19:327–331.
Article7. Rustin GJ. Can we now agree to use the same definition to measure response according to CA-125? J Clin Oncol. 2004; 22:4035–4036.
Article8. Lee CK, Friedlander M, Brown C, Gebski VJ, Georgoulopoulos A, Vergote I, et al. Early decline in cancer antigen 125 as a surrogate for progression-free survival in recurrent ovarian cancer. J Natl Cancer Inst. 2011; 103:1338–1342.
Article9. Colakovic´ S, Lukiç V, Mitroviç L, Jeliç S, Susnjar S, Marinkoviç J. Prognostic value of CA125 kinetics and half-life in advanced ovarian cancer. Int J Biol Markers. 2000; 15:147–152.
Article10. Gadducci A, Cosio S, Fanucchi A, Negri S, Cristofani R, Genazzani AR. The predictive and prognostic value of serum CA 125 half-life during paclitaxel/platinum-based chemotherapy in patients with advanced ovarian carcinoma. Gynecol Oncol. 2004; 93:131–136.
Article11. Riedinger JM, Eche N, Basuyau JP, Dalifard I, Hacene K, Pichon MF. Prognostic value of serum CA 125 bi-exponential decrease during first line paclitaxel/platinum chemotherapy: a French multicentric study. Gynecol Oncol. 2008; 109:194–198.
Article12. Riedinger JM, Wafflart J, Ricolleau G, Eche N, Larbre H, Basuyau JP, et al. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: results of a French multicentric study. Ann Oncol. 2006; 17:1234–1238.
Article13. Mano A, Falcão A, Godinho I, Santos J, Leitão F, Oliveira C, et al. CA-125 AUC as a new prognostic factor for patients with ovarian cancer. Gynecol Oncol. 2005; 97:529–534.
Article14. Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, et al. Re: new guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). J Natl Cancer Inst. 2004; 96:487–488.
Article15. Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010; 28:3323–3329.
Article16. Nustad K, Bast RC Jr, Brien TJ, Nilsson O, Seguin P, Suresh MR, et al. International Society for Oncodevelopmental Biology and Medicine. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 workshop. Tumour Biol. 1996; 17:196–219.
Article17. Høgdall EV, Christensen L, Kjaer SK, Blaakaer J, Kjaerbye-Thygesen A, Gayther S, et al. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients. From the danish "MALOVA" ovarian cancer study. Gynecol Oncol. 2007; 104:508–515.
Article18. Changes in definitions of clinical staging for carcinoma of the cervix and ovary: International Federation of Gynecology and Obstetrics. Am J Obstet Gynecol. 1987; 156:263–264.19. Böcker W. [WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics]. Verh Dtsch Ges Pathol. 2002; 86:116–119.20. Gadducci A, Zola P, Landoni F, Maggino T, Sartori E, Bergamino T, et al. Serum half-life of CA 125 during early chemotherapy as an independent prognostic variable for patients with advanced epithelial ovarian cancer: results of a multicentric Italian study. Gynecol Oncol. 1995; 58:42–47.
Article21. Buller RE, Berman ML, Bloss JD, Manetta A, DiSaia PJ. CA 125 regression: a model for epithelial ovarian cancer response. Am J Obstet Gynecol. 1991; 165:360–367.
Article22. Buller RE, Berman ML, Bloss JD, Manetta A, DiSaia PJ. Serum CA125 regression in epithelial ovarian cancer: correlation with reassessment findings and survival. Gynecol Oncol. 1992; 47:87–92.
Article23. Riedinger JM, Bonnetain F, Basuyau JP, Eche N, Larbre H, Dalifard I, et al. Change in CA 125 levels after the first cycle of induction chemotherapy is an independent predictor of epithelial ovarian tumour outcome. Ann Oncol. 2007; 18:881–885.
Article24. Paramasivam S, Tripcony L, Crandon A, Quinn M, Hammond I, Marsden D, et al. Prognostic importance of preoperative CA-125 in International Federation of Gynecology and Obstetrics stage I epithelial ovarian cancer: an Australian multicenter study. J Clin Oncol. 2005; 23:5938–5942.
Article25. Vorgias G, Iavazzo C, Savvopoulos P, Myriokefalitaki E, Katsoulis M, Kalinoglou N, et al. Can the preoperative Ca-125 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? A single institution cohort study. Gynecol Oncol. 2009; 112:11–15.
Article26. Zorn KK, Tian C, McGuire WP, Hoskins WJ, Markman M, Muggia FM, et al. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: a Gynecologic Oncology Group study. Cancer. 2009; 115:1028–1035.
Article27. Wong C, Dai ZM, Lele SB, Natarajan N. Comparison of CA 125 after three courses of chemotherapy and results of second-look surgery. Eur J Gynaecol Oncol. 2000; 21:70–73.28. Le T, Faught W, Hopkins L, Fung-Kee-Fung M. Importance of CA125 normalization during neoadjuvant chemotherapy followed by planned delayed surgical debulking in patients with epithelial ovarian cancer. J Obstet Gynaecol Can. 2008; 30:665–670.
Article29. Tate S, Hirai Y, Takeshima N, Hasumi K. CA125 regression during neoadjuvant chemotherapy as an independent prognostic factor for survival in patients with advanced ovarian serous adenocarcinoma. Gynecol Oncol. 2005; 96:143–149.
Article30. Kang S, Seo SS, Park SY. Nadir CA-125 level is an independent prognostic factor in advanced epithelial ovarian cancer. J Surg Oncol. 2009; 100:244–247.
Article31. Liu PY, Alberts DS, Monk BJ, Brady M, Moon J, Markman M. An early signal of CA-125 progression for ovarian cancer patients receiving maintenance treatment after complete clinical response to primary therapy. J Clin Oncol. 2007; 25:3615–3620.
Article32. Rustin GJ, Nelstrop AE, McClean P, Brady MF, McGuire WP, Hoskins WJ, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996; 14:1545–1551.
Article33. Bhoola SM, Coleman RL, Herzog T, Morris R, Bryant C, Estes JM, et al. Retrospective analysis of weekly topotecan as salvage therapy in relapsed ovarian cancer. Gynecol Oncol. 2004; 95:564–569.
Article34. Bodnar L, Wcislo G, Nasilowska A, Szarlej-Wcislo K, Gasowska-Bodnar A, Smoter M, et al. Salvage therapy with topotecan in heavily pretreated ovarian cancer patients. J Cancer Res Clin Oncol. 2009; 135:815–821.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validity of serum CA125 level for assessing responsiveness to combined chemotherapy in epithelial ovarian cancer
- Prognostic value of serum CA-125 in patients with advanced epithelial ovarian cancer followed by complete remission after adjuvant chemotherapy
- The effectiveness of CA125 and HE4 as clinical prognostic markers in epithelial ovarian cancer patients with BRCA mutation
- Potential predictors for chemotherapeutic response and prognosis in epithelial ovarian, fallopian tube and primary peritoneal cancer patients treated with platinum-based chemotherapy
- Treatment strategies for patients with advanced ovarian cancer undergoing neoadjuvant chemotherapy: interval debulking surgery or additional chemotherapy?