Yonsei Med J.
2016 Mar;57(2):441-448. 10.3349/ymj.2016.57.2.441.
Transplantation of a Scaffold-Free Cartilage Tissue Analogue for the Treatment of Physeal Cartilage Injury of the Proximal Tibia in Rabbits
- Affiliations
-
- 1Department of Orthopaedic Surgery, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea.
- 2Department of Orthopaedic Surgery, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea. changhoonj@yahoo.com
- KMID: 2374051
- DOI: http://doi.org/10.3349/ymj.2016.57.2.441
Abstract
- PURPOSE
The purpose of this study was to investigate the effects of transplantation of an in vitro-generated, scaffold-free, tissue-engineered cartilage tissue analogue (CTA) using a suspension chondrocyte culture in a rabbit growth-arrest model.
MATERIALS AND METHODS
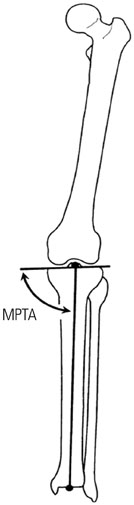
We harvested cartilage cells from the articular cartilage of the joints of white rabbits and made a CTA using a suspension culture of 2x107 cells/mL. An animal growth plate defect model was made on the medial side of the proximal tibial growth plate of both tibias of 6-week-old New Zealand white rabbits (n=10). The allogenic CTA was then transplanted onto the right proximal tibial defect. As a control, no implantation was performed on the left-side defect. Plain radiographs and the medial proximal tibial angle were obtained at 1-week intervals for evaluation of bone bridge formation and the degree of angular deformity until postoperative week 6. We performed a histological evaluation using hematoxylin-eosin and Alcian blue staining at postoperative weeks 4 and 6.
RESULTS
Radiologic study revealed a median medial proximal tibial angle of 59.0degrees in the control group and 80.0degrees in the CTA group at 6 weeks. In the control group, statistically significant angular deformities were seen 3 weeks after transplantation (p<0.05). On histological examination, the transplanted CTA was maintained in the CTA group at 4 and 6 weeks postoperative. Bone bridge formation was observed in the control group.
CONCLUSION
In this study, CTA transplantation minimized deformity in the rabbit growth plate injury model, probably via the attenuation of bone bridge formation.
Keyword
MeSH Terms
-
Animals
*Bone Transplantation
Cartilage/anatomy & histology
Cell Culture Techniques
Cells, Cultured
Chondrocytes/*cytology/transplantation
Growth Plate/anatomy & histology/*surgery
*Mesenchymal Stem Cell Transplantation
Rabbits
Tibia/*surgery
Tissue Engineering
Transplantation, Autologous/methods
Transplantation, Homologous
Figure
Reference
-
1. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981; 1:3–11.
Article2. Lee EH, Gao GX, Bose K. Management of partial growth arrest: physis, fat, or silastic? J Pediatr Orthop. 1993; 13:368–372.3. Langenskiöld A. An operation for partial closure of an epiphysial plate in children, and its experimental basis. J Bone Joint Surg Br. 1975; 57:325–330.
Article4. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989; 71:13–16.
Article5. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990; 10:769–776.6. Foster BK, John B, Hasler C. Free fat interpositional graft in acute physeal injuries: the anticipatory Langenskiöld procedure. J Pediatr Orthop. 2000; 20:282–285.
Article7. Bright RW. Operative correction of partial epiphyseal plate closure by osseous-bridge resection and silicone-rubber implant. An experimental study in dogs. J Bone Joint Surg Am. 1974; 56:655–664.
Article8. Macksoud WS, Bright R. Bar resection and silastic interposition in distal radial physeal arrest. Orthop Trans. 1989; 13:1–2.9. Olin A, Creasman C, Shapiro F. Free physeal transplantation in the rabbit. An experimental approach to focal lesions. J Bone Joint Surg Am. 1984; 66:7–20.
Article10. Bowen CV, Ethridge CP, O'Brien BM, Frykman GK, Gumley GJ. Experimental microvascular growth plate transfers. Part I--Investigation of vascularity. J Bone Joint Surg Br. 1988; 70:305–310.
Article11. Foster BK, Hansen AL, Gibson GJ, Hopwood JJ, Binns GF, Wiebkin OW. Reimplantation of growth plate chondrocytes into growth plate defects in sheep. J Orthop Res. 1990; 8:555–564.
Article12. Tobita M, Ochi M, Uchio Y, Mori R, Iwasa J, Katsube K, et al. Treatment of growth plate injury with autogenous chondrocytes: a study in rabbits. Acta Orthop Scand. 2002; 73:352–358.13. Chung R, Foster BK, Xian CJ. Preclinical studies on mesenchymal stem cell-based therapy for growth plate cartilage injury repair. Stem Cells Int. 2011; 2011:570125.
Article14. Campbell CJ, Grisolia A, Zanconato G. The effects produced in the cartilaginous epiphyseal plate of immature dogs by experimental surgical traumata. J Bone Joint Surg Am. 1959; 41-A:1221–1242.
Article15. Kraft JJ, Jeong C, Novotny JE, Seacrist T, Chan G, Domzalski M, et al. Effects of hydrostatic loading on a self-aggregating, suspension culture-derived cartilage tissue analog. Cartilage. 2011; 2:254–264.
Article16. Novotny JE, Turka CM, Jeong C, Wheaton AJ, Li C, Presedo A, et al. Biomechanical and magnetic resonance characteristics of a cartilage-like equivalent generated in a suspension culture. Tissue Eng. 2006; 12:2755–2764.
Article17. Mohanraj B, Farran AJ, Mauck RL, Dodge GR. Time-dependent functional maturation of scaffold-free cartilage tissue analogs. J Biomech. 2014; 47:2137–2142.
Article18. Estrada LE, Dodge GR, Richardson DW, Farole A, Jimenez SA. Characterization of a biomaterial with cartilage-like properties expressing type X collagen generated in vitro using neonatal porcine articular and growth plate chondrocytes. Osteoarthritis Cartilage. 2001; 9:169–177.
Article19. Yoshida K, Higuchi C, Nakura A, Nakamura N, Yoshikawa H. Treatment of partial growth arrest using an in vitro-generated scaffold-free tissue-engineered construct derived from rabbit synovial mesenchymal stem cells. J Pediatr Orthop. 2012; 32:314–321.
Article20. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994; 25:425–465.
Article21. Lennox DW, Goldner RD, Sussman MD. Cartilage as an interposition material to prevent transphyseal bone bridge formation: an experimental model. J Pediatr Orthop. 1983; 3:207–210.
Article22. Lee EH, Chen F, Chan J, Bose K. Treatment of growth arrest by transfer of cultured chondrocytes into physeal defects. J Pediatr Orthop. 1998; 18:155–160.
Article23. Kawabe N, Ehrlich MG, Mankin HJ. Growth plate reconstruction using chondrocyte allograft transplants. J Pediatr Orthop. 1987; 7:381–388.
Article24. Wakitani S, Kimura T, Hirooka A, Ochi T, Yoneda M, Yasui N, et al. Repair of rabbit articular surfaces with allograft chondrocytes embedded in collagen gel. J Bone Joint Surg Br. 1989; 71:74–80.
Article25. Steinwachs M. New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy. 2009; 25:208–211.
Article26. O'Grady JE, Bordon DM. Global regulatory registration requirements for collagen-based combination products: points to consider. Adv Drug Deliv Rev. 2003; 55:1699–1721.27. Reginato AM, Iozzo RV, Jimenez SA. Formation of nodular structures resembling mature articular cartilage in long-term primary cultures of human fetal epiphyseal chondrocytes on a hydrogel substrate. Arthritis Rheum. 1994; 37:1338–1349.
Article28. Ebihara G, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, et al. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials. 2012; 33:3846–3851.
Article29. Planka L, Srnec R, Rauser P, Stary D, Filova E, Jancar J, et al. Nanotechnology and mesenchymal stem cells with chondrocytes in prevention of partial growth plate arrest in pigs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012; 156:128–134.
Article30. Tobita M, Ochi M, Uchio Y, Mori R, Iwasa J, Katsube K, et al. Treatment of growth plate injury with autogenous chondrocytes: a study in rabbits. Acta Orthop Scand. 2002; 73:352–358.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Physeal Defect Model of Rabbit's Tibia : Conventional Curettage vs Box Osteotome Method
- Conservative Treatment of Physeal Bar after Triradiate Cartilage Injury
- Clinical Results of Transplantation of Tissue-Engineered Cartilage and Future Direction of Cartilage Repair: Novel Approach with Minimally Invasive Procedure
- Cultured Chondrocyte Transplantation in the Damaged Growth Plate
- Atelocollagen Scaffold Enhances Cartilage Regeneration in Osteochondral Defects: A Study in Rabbits