J Korean Med Sci.
2016 Jun;31(6):983-988. 10.3346/jkms.2016.31.6.983.
Relaxin Receptor RXFP1 and RXFP2 Expression in Ligament, Tendon, and Shoulder Joint Capsule of Rats
- Affiliations
-
- 1Division of Physical Medicine and Rehabilitation, Department of Orthopaedic Surgery, Stanford University, Stanford, CA, USA. rehabmfred2015@gmail.com
- 2Department of Physical Medicine & Rehabilitation, Eulji University Hospital and Eulji University School of Medicine, Daejeon, Korea.
- 3Eulji Medi-Bio Research Institute, Daejeon, Korea.
- 4Department of Biochemistry, Eulji University School of Medicine, Daejeon, Korea.
- 5Department of Pathology, Eulji University School of Medicine, Daejeon, Korea.
- KMID: 2373710
- DOI: http://doi.org/10.3346/jkms.2016.31.6.983
Abstract
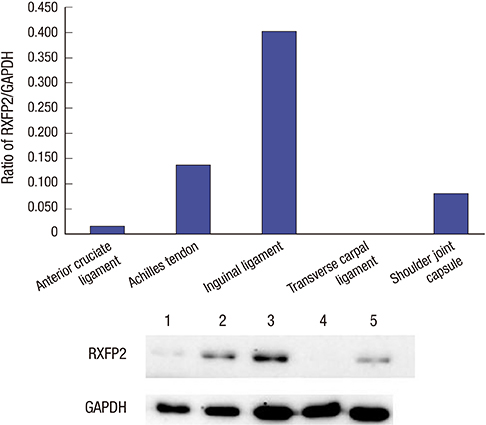
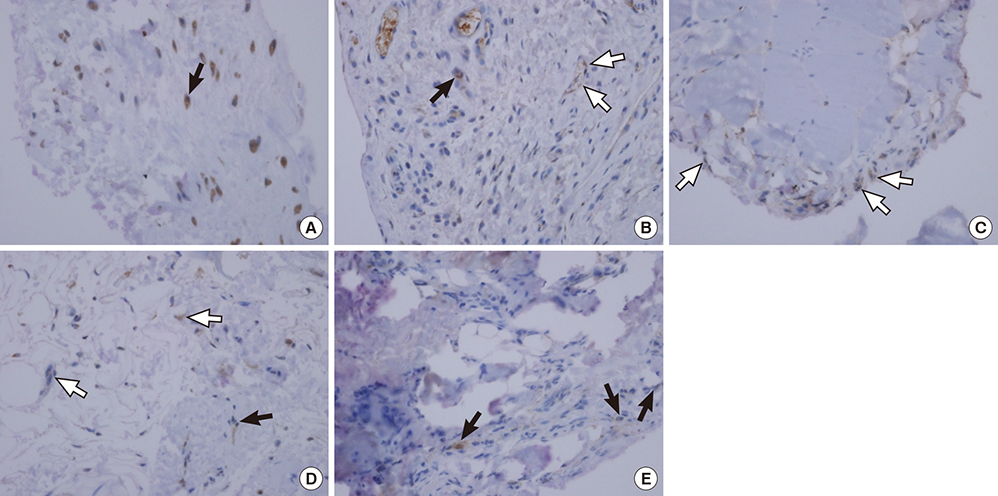
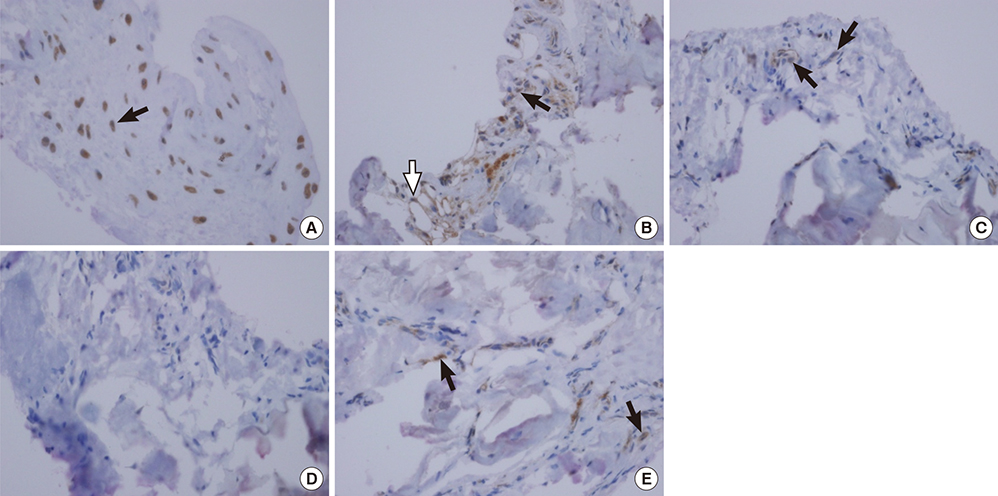
- Numerous musculoskeletal disorders are caused by thickened ligament, tendon stiffness, or fibrosis of joint capsule. Relaxin, a peptide hormone, can exert collagenolytic effect on ligamentous and fibrotic tissues. We hypothesized that local injection of relaxin could be used to treat entrapment neuropathy and adhesive capsulitis. Because hormonal effect depends on the receptor of the hormone on the target cell, it is important to confirm the presence of such hormonal receptor at the target tissue before the hormone therapy is initiated. The aim of this study was to determine whether there were relaxin receptors in the ligament, tendon, and joint capsular tissues of rats and to identify the distribution of relaxin receptors in these tissues. Transverse carpal ligaments (TCLs), inguinal ligaments, anterior cruciate ligaments (ACLs), Achilles tendons, and shoulder joint capsules were obtained from male Wistar rats. Western blot analysis was used to identify relaxin receptor isoforms RXFP1 and RXFP2. The distribution of relaxin receptors was determined by immunohistochemical staining. The RXFP1 isoform was found in all tissues examined. The RXFP2 isoform was present in all tissues but the TCLs. Its expression in ACLs tissues was relatively weak compared to that in other tissues. Our results revealed that RXFP1 and RXFP2 were distributed in distinctly different patterns according to the type of tissue (vascular endothelial cells, fibroblast-like cells) they were identified.
Keyword
MeSH Terms
Figure
Reference
-
1. Rempel DM, Diao E. Entrapment neuropathies: pathophysiology and pathogenesis. J Electromyogr Kinesiol. 2004; 14:71–75.2. Neviaser AS, Neviaser RJ. Adhesive capsulitis of the shoulder. J Am Acad Orthop Surg. 2011; 19:536–542.3. Bouche P. Compression and entrapment neuropathies. Handb Clin Neurol. 2013; 115:311–366.4. Bennett RG. Relaxin and its role in the development and treatment of fibrosis. Transl Res. 2009; 154:1–6.5. Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. Activation of orphan receptors by the hormone relaxin. Science. 2002; 295:671–674.6. Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev. 2006; 58:7–31.7. Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol. 1999; 13:681–691.8. Dehghan F, Muniandy S, Yusof A, Salleh N. Sex-steroid regulation of relaxin receptor isoforms (RXFP1 & RXFP2) expression in the patellar tendon and lateral collateral ligament of female WKY rats. Int J Med Sci. 2014; 11:180–191.9. Relaxin WG. Annu Rev Physiol. 1984; 46:43–52.10. Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013; 93:405–480.11. Scott DJ, Rosengren KJ, Bathgate RA. The different ligand-binding modes of relaxin family peptide receptors RXFP1 and RXFP2. Mol Endocrinol. 2012; 26:1896–1906.12. Scott DJ, Layfield S, Riesewijk A, Morita H, Tregear GW, Bathgate RA. Characterization of the mouse and rat relaxin receptors. Ann N Y Acad Sci. 2005; 1041:8–12.13. McGowan BM, Stanley SA, Donovan J, Thompson EL, Patterson M, Semjonous NM, Gardiner JV, Murphy KG, Ghatei MA, Bloom SR. Relaxin-3 stimulates the hypothalamic-pituitary-gonadal axis. Am J Physiol Endocrinol Metab. 2008; 295:E278–86.14. Belgi A, Hossain MA, Shabanpoor F, Chan L, Zhang S, Bathgate RA, Tregear GW, Wade JD. Structure and function relationship of murine insulin-like peptide 5 (INSL5): free C-terminus is essential for RXFP4 receptor binding and activation. Biochemistry. 2011; 50:8352–8361.15. Dragoo JL, Lee RS, Benhaim P, Finerman GA, Hame SL. Relaxin receptors in the human female anterior cruciate ligament. Am J Sports Med. 2003; 31:577–584.16. Lubahn J, Ivance D, Konieczko E, Cooney T. Immunohistochemical detection of relaxin binding to the volar oblique ligament. J Hand Surg Am. 2006; 31:80–84.17. Clifton KB, Rodner C, Wolf JM. Detection of relaxin receptor in the dorsoradial ligament, synovium, and articular cartilage of the trapeziometacarpal joint. J Orthop Res. 2014; 32:1061–1067.18. Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, Doty KD, Debrah DO, Shroff SG, Conrad KP. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J. 2006; 20:2352–2362.19. Soh YM, Tiwari A, Mahendroo M, Conrad KP, Parry LJ. Relaxin regulates hyaluronan synthesis and aquaporins in the cervix of late pregnant mice. Endocrinology. 2012; 153:6054–6064.20. Masterson R, Hewitson TD, Kelynack K, Martic M, Parry L, Bathgate R, Darby I, Becker G. Relaxin down-regulates renal fibroblast function and promotes matrix remodelling in vitro. Nephrol Dial Transplant. 2004; 19:544–552.21. Chow BS, Chew EG, Zhao C, Bathgate RA, Hewitson TD, Samuel CS. Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: the additional involvement of iNOS. PLoS One. 2012; 7:e42714.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reconstruction of Posterior Cruciate Ligament Using Semitendinosus Tendon (3 cases)
- Intraarticular Ganglion Arising from the Posterior Cruciate Ligament
- The Reconstruction of Anterior Cruciate Ligament Using Patellar Tendon under Arthroscopy
- Expression of Estrogen Receptor on Ligamentum Flavum and Facet Joint Capsule in Degenerative Spinal Stenosis
- Bilateral Congenital Absence of the Long Head of Biceps Brachii Tendon in Shoulder Joints: A case report