Ann Lab Med.
2016 Nov;36(6):595-598. 10.3343/alm.2016.36.6.595.
Reference Intervals for Plasma Amyloid β in Korean Adults Without Cognitive Impairment
- Affiliations
-
- 1Department of Family Practice & Community Health, Ajou University School of Medicine, Suwon, Korea.
- 2Department of Clinical Pharmacology and Therapeutics, CHA Bundang Medical Center, CHA University, Seongnam, Korea. drdooycho@gmail.com
- KMID: 2373599
- DOI: http://doi.org/10.3343/alm.2016.36.6.595
Abstract
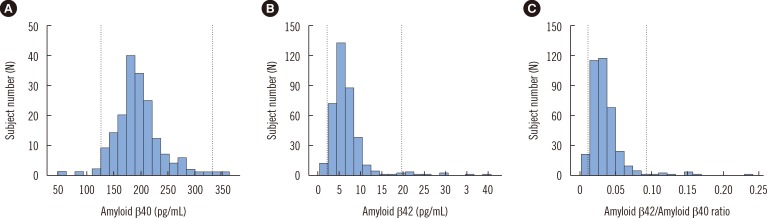
- Amyloid β (Aβ) peptides are important components of plaques in patients with Alzheimer's disease (AD). Recent studies suggest that a low plasma ratio of Aβ42 to Aβ40 may precede the development of the sporadic form of AD. The aim of this study was to establish reference intervals for plasma Aβ in Korean adults. A total of 370 apparently healthy individuals (181 males and 189 females aged 40-69 yr) without cognitive impairment were enrolled. Plasma concentrations of Aβ40 and Aβ42 were measured by using a human amyloid β assay kit (Immuno-Biological Laboratories, Japan). Reference intervals were established according to the "CLSI guidelines for defining, establishing, and verifying reference intervals in the clinical laboratory". There was no need to partition the data with respect to gender or age group. The 95th percentile reference intervals for Aβ40 and Aβ42 were 127-331 pg/mL and 2.31-19.84 pg/mL, respectively. The reference interval for the Aβ42/Aβ40 ratio was 0.011-0.092. Plasma Aβ concentrations obtained in this study could be used as reference intervals for clinical purposes.
MeSH Terms
Figure
Reference
-
1. Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc. 2003; 78:1290–1308. PMID: 14531488.2. Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001; 56:1143–1153. PMID: 11342678.3. Varon D, Loewenstein DA, Potter E, Greig MT, Agron J, Shen Q, et al. Minimal atrophy of the entorhinal cortex and hippocampus: progression of cognitive impairment. Dement Geriatr Cogn Disord. 2011; 31:276–283. PMID: 21494034.4. Gaugler JE, Kane RL, Johnston JA, Sarsour K. Sensitivity and specificity of diagnostic accuracy in Alzheimer’s disease: a synthesis of existing evidence. Am J Alzheimers Dis Other Demen. 2013; 28:337–347. PMID: 23687179.5. Vassar R. BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci. 2004; 23:105–114. PMID: 15126696.6. Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, et al. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001; 58:373–379. PMID: 11255440.7. Toledo JB, Shaw LM, Trojanowski JQ. Plasma amyloid beta measurements - a desired but elusive Alzheimer's disease biomarker. Alzheimers Res Ther. 2013; 5:8. PMID: 23470128.8. Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, et al. Association of low plasma Aβ42/Aβ40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007; 64:354–362. PMID: 17353377.9. Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011; 305:261–266. PMID: 21245181.10. Park JH, Park YN, Ko HJ. Modification of the mini-mental state examination for use with the elderly in a non-western society. Part II: cutoff points and their diagnostic validities. Int J Geriatr Psychiatry. 1991; 6:875–882.11. Hata S, Fujishige S, Araki Y, Kato N, Araseki M, Nishimura M, et al. Alcadein cleavages by amyloid beta-precursor protein (APP) alpha- and gamma-secretases generate small peptides, p3-Alcs, indicating Alzheimer disease-related gamma-secretase dysfunction. J Biol Chem. 2009; 284:36024–36033. PMID: 19864413.12. Ray B, Banerjee PK, Greig NH, Lahiri DK. Memantine treatment decreases levels of secreted Alzheimer’s amyloid precursor protein (APP) and amyloid beta (Aβ) peptide in the human neuroblastoma cells. Neurosci Lett. 2010; 470:1–5. PMID: 19948208.13. Horowitz GL, Altaie S, Boyd JC, Ceriotti F, Garg U, Horn P, et al. Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guideline. EP28-A3c. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2010.14. Sjögren M, Vanderstichele H, Agren H, Zachrisson O, Edsbagge M, Wikkelsø C, et al. Tau and Aβ42 in cerebrospinal fluid from healthy adults 21-93 years of age: establishment of reference values. Clin Chem. 2001; 47:1776–1781. PMID: 11568086.15. Burkhard PR, Fournier R, Mermillod B, Krause KH, Bouras C, Irminger I. Cerebrospinal fluid tau and Aβ42 concentrations in healthy subjects: delineation of reference intervals and their limitations. Clin Chem Lab Med. 2004; 42:396–407. PMID: 15147150.16. Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback WM, et al. Aβ peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009; 5:18–29. PMID: 19118806.17. Kuo YM, Kokjohn TA, Kalback W, Luehrs D, Galasko DR, Chevallier N, et al. Amyloid-β peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem Biophys Res Commun. 2000; 268:750–756. PMID: 10679277.18. Head E, Doran E, Nistor M, Hill M, Schmitt FA, Haier RJ, et al. Plasma amyloid-β as a function of age, level of intellectual disability, and presence of dementia in Down syndrome. J Alzheimers Dis. 2011; 23:399–409. PMID: 21116050.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Different Associations of Plasma Biomarkers in Alzheimer's Disease, Mild Cognitive Impairment, Vascular Dementia, and Ischemic Stroke
- Correlation between Sleep and C-reactive Protein of Patients in Amnestic Mild Cognitive Impairment and Alzheimer’s Dementia
- Justicidin A Reduces β-Amyloid via Inhibiting Endocytosis of β-Amyloid Precursor Protein
- A Voxel Wise Analysis of Cerebral Beta Amyloid Retention in Healthy Controls and Subjects with Amnestic Mild Cognitive Impairment and Alzheimer's Disease
- Effects of Cognitive-based Interventions of Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-analysis