Ann Lab Med.
2016 Nov;36(6):505-512. 10.3343/alm.2016.36.6.505.
Current Pathological and Laboratory Considerations in the Diagnosis of Disseminated Intravascular Coagulation
- Affiliations
-
- 1Institute of Infection and Global Health, University of Liverpool, Liverpool, United Kingdom. Toh@liverpool.ac.uk
- 2Roald Dahl Haemostasis & Thrombosis Centre, Royal Liverpool University Hospital, Liverpool, United Kingdom.
- KMID: 2373587
- DOI: http://doi.org/10.3343/alm.2016.36.6.505
Abstract
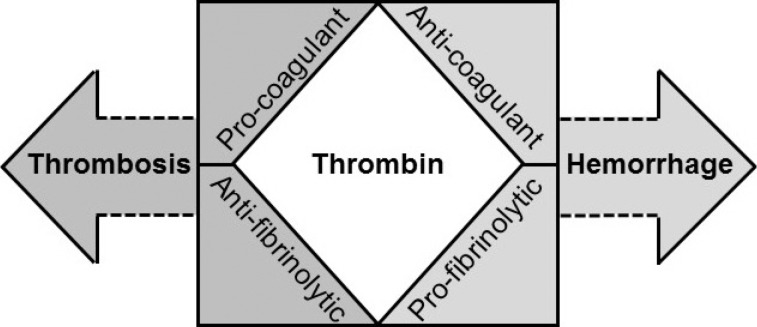
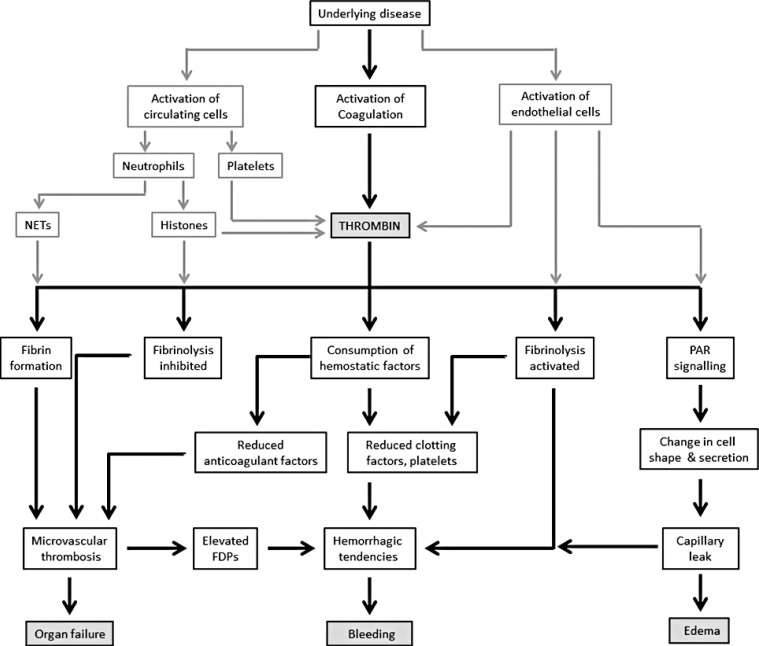
- Systemically sustained thrombin generation in vivo is the hallmark of disseminated intravascular coagulation (DIC). Typically, this is in response to a progressing disease state that is associated with significant cellular injury. The etiology could be infectious or noninfectious, with the main pathophysiological mechanisms involving cross-activation among coagulation, innate immunity, and inflammatory responses. This leads to consumption of both pro- and anticoagulant factors as well as endothelial dysfunction and disrupted homeostasis at the blood vessel wall interface. In addition to the release of tissue plasminogen activator (tPA) and soluble thrombomodulin (sTM) following cellular activation and damage, respectively, there is the release of damage-associated molecular patterns (DAMPs) such as extracellular histones and cell-free DNA. Extracellular histones are increasingly recognized as significantly pathogenic in critical illnesses through direct cell toxicity, the promotion of thrombin generation, and the induction of neutrophil extracellular trap (NET) formation. Clinically, high circulating levels of histones and histone-DNA complexes are associated with multiorgan failure, DIC, and adverse patient outcomes. Their measurements as well as that of other DAMPs and molecular markers of thrombin generation are not yet applicable in the routine diagnostic laboratory. To provide a practical diagnostic tool for acute DIC, a composite scoring system using rapidly available coagulation tests is recommended by the International Society on Thrombosis and Haemostasis. Its usefulness and limitations are discussed alongside the advances and unanswered questions in DIC pathogenesis.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Acquired Hemophilia A with Gastrointestinal Bleeding
Narae Park, Jin Seok Jang, Jae Hwang Cha
Clin Endosc. 2020;53(1):90-93. doi: 10.5946/ce.2019.036.
Reference
-
1. Crawley JT, Zanardelli S, Chion CK, Lane DA. The central role of thrombin in hemostasis. J Thromb Haemost. 2007; 5(S1):95–101. PMID: 17635715.2. Taylor FB Jr, Wada H, Kinasewitz G. Description of compensated and uncompensated disseminated intravascular coagulation (DIC) responses (non-overt and overt DIC) in baboon models of intravenous and intraperitoneal Escherichia coli sepsis and in the human model of endotoxemia: toward a better definition of DIC. Crit Care Med. 2000; 28(9S):S12–S19. PMID: 11007191.3. Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001; 86:1327–1330. PMID: 11816725.4. Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004; 32:2416–2421. PMID: 15599145.5. Kjalke M, Monroe DM, Hoffman M, Oliver JA, Ezban M, Roberts HR. Active site-inactivated factors VIIa, Xa, and IXa inhibit individual steps in a cell-based model of tissue factor-initiated coagulation. Thromb Haemost. 1998; 80:578–584. PMID: 9798973.6. Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010; 17:2059–2069. PMID: 20423310.7. Levin EG, Marzec U, Anderson J, Harker LA. Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J Clin Invest. 1984; 74:1988–1995. PMID: 6542570.8. Gelehrter TD, Sznycer-Laszuk R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. J Clin Invest. 1986; 77:165–169. PMID: 2418059.9. Wang W, Boffa MB, Bajzar L, Walker JB, Nesheim ME. A study of the mechanism of inhibition of fibrinolysis by activated thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1998; 273:27176–27181. PMID: 9765237.10. Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwège V, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994; 153:3245–3255. PMID: 7522256.11. Taylor FB Jr, Hoogendoorn H, Chang AC, Peer G, Nesheim ME, Catlett R, et al. Anticoagulant and fibrinolytic activities are promoted, not retarded, in vivo after thrombin generation in the presence of a monoclonal antibody that inhibits activation of protein C. Blood. 1992; 79:1720–1728. PMID: 1558968.12. Aird WC. Endothelium and haemostasis. Hamostaseologie. 2015; 35:11–16. PMID: 25666572.13. Monahan-Earley R, Dvorak AM, Aird WC. Evolutionary origins of the blood vascular system and endothelium. J Thromb Haemost. 2013; 11(Sl):46–66. PMID: 23809110.14. Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002; 296:1880–1882. PMID: 12052963.15. Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005; 115:1267–1274. PMID: 15841214.16. Moxon CA, Chisala NV, Mzikamanda R, MacCormick I, Harding S, Downey C, et al. Laboratory evidence of disseminated intravascular coagulation is associated with a fatal outcome in children with cerebral malaria despite an absence of clinically evident thrombosis or bleeding. J Thromb Haemost. 2015; 13:1653–1664. PMID: 26186686.17. Moxon CA, Wassmer SC, Milner DA Jr, Chisala NV, Taylor TE, Seydel KB, et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood. 2013; 122:842–851. PMID: 23741007.18. Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013; 498:502–505. PMID: 23739325.19. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013; 13:34–45. PMID: 23222502.20. Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011; 9(S1):182–188. PMID: 21781254.21. Ito T. PAMPs and DAMPs as triggers for DIC. J Intensive Care. 2014; 2:67. PMID: 25705424.22. Conway EM. Reincarnation of ancient links between coagulation and complement. J Thromb Haemost. 2015; 13(S1):S121–S132. PMID: 26149013.23. Kim JE, Lee N, Gu JY, Yoo HJ, Kim HK. Circulating levels of DNA-histone complex and dsDNA are independent prognostic factors of disseminated intravascular coagulation. Thromb Res. 2015; 135:1064–1069. PMID: 25843168.24. Nakahara M, Ito T, Kawahara K, Yamamoto M, Nagasato T, Shrestha B, et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS One. 2013; 8:e75961. PMID: 24098750.25. Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013; 187:160–169. PMID: 23220920.26. Alhamdi Y, Zi M, Abrams ST, Liu T, Su D, Welters I, et al. Circulating histone concentrations differentially affect the predominance of left or right ventricular dysfunction in critical illness. Crit Care Med. 2016; 44:e278–e288. PMID: 26588828.27. Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011; 118:3708–3714. PMID: 21700775.28. Longstaff C, Varjú I, Sótonyi P, Szabó L, Krumrey M, Hoell A, et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013; 288:6946–6956. PMID: 23293023.29. Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014; 123:2768–2776. PMID: 24366358.30. Yamamichi S, Fujiwara Y, Kikuchi T, Nishitani M, Matsushita Y, Hasumi K. Extracellular histone induces plasma hyaluronan-binding protein (factor VII activating protease) activation in vivo. Biochem Biophys Res Commun. 2011; 409:483–488. PMID: 21600885.31. Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011; 118:1952–1961. PMID: 21673343.32. Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011; 9:1795–1803. PMID: 21711444.33. Barranco-Medina S, Pozzi N, Vogt AD, Di Cera E. Histone H4 promotes prothrombin autoactivation. J Biol Chem. 2013; 288:35749–35757. PMID: 24178300.34. Alhamdi Y, Abrams ST, Cheng Z, Jing S, Su D, Liu Z, et al. Circulating histones are major mediators of cardiac injury in patients with sepsis. Crit Care Med. 2015; 43:2094–2103. PMID: 26121070.35. Levi M, Toh C, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009; 145:24–33. PMID: 19222477.36. Toh CH, Hoots WK. SSC on Disseminated Intravascular Coagulation of the ISTH. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost. 2007; 5:604–606. PMID: 17096704.37. Gando S, Saitoh D, Ogura H, Fujishima S, Mayumi T, Araki T, et al. A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit Care. 2013; 17:R111. PMID: 23787004.38. Wada H, Gabazza EC, Asakura H, Koike K, Okamoto K, Maruyama I, et al. Comparison of diagnostic criteria for disseminated intravascular coagulation (DIC): diagnostic criteria of the International Society of Thrombosis and Hemostasis and of the Japanese Ministry of Health and Welfare for overt DIC. Am J Hematol. 2003; 74:17–22. PMID: 12949885.39. Toh CH, Downey C. Performance and prognostic importance of a new clinical and laboratory scoring system for identifying non-overt disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2005; 16:69–74. PMID: 15650549.40. Kinasewitz GT, Zein JG, Lee GL, Nazir SA, Taylor FB Jr. Prognostic value of a simple evolving disseminated intravascular coagulation score in patients with severe sepsis. Crit Care Med. 2005; 33:2214–2221. PMID: 16215373.41. Dhainaut JF, Shorr AF, Macias WL, Kollef MJ, Levi M, Reinhart K, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med. 2005; 33:341–348. PMID: 15699837.42. Levi M, Meijers JC. DIC: which laboratory tests are most useful. Blood Rev. 2011; 25:33–37. PMID: 20950905.43. Kitchen S, Cartwright I, Woods TA, Jennings I, Preston FE. Lipid composition of seven APTT reagents in relation to heparin sensitivity. Br J Haematol. 1999; 106:801–808. PMID: 10468876.44. Wada H, Thachil J, Di Nisio M, Mathew P, Kurosawa S, Gando S, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013; 11:761–767.45. Kim HK, Hong KH, Toh CH. Scientific and Standardization Committee on DIC of The International Society on Thrombosis and Haemostasis. Application of the international normalized ratio in the scoring system for disseminated intravascular coagulation. J Thromb Haemost. 2010; 8:1116–1118. PMID: 20230426.46. Thiele T, Selleng K, Selleng S, Greinacher A, Bakchoul T. Thrombocytopenia in the intensive care unit-diagnostic approach and management. Semin Hematol. 2013; 50:239–250. PMID: 23953341.47. Kim SY, Kim JE, Kim HK, Han KS, Toh CH. Accuracy of platelet counting by automated hematologic analyzers in acute leukemia and disseminated intravascular coagulation: potential effects of platelet activation. Am J Clin Pathol. 2010; 134:634–647. PMID: 20855645.48. Yaguchi A, Lobo FL, Vincent JL, Pradier O. Platelet function in sepsis. J Thromb Haemost. 2004; 2:2096–2102. PMID: 15613012.49. Hyun J, Kim HK, Kim JE, Lim MG, Jung JS, Park S, et al. Correlation between plasma activity of ADAMTS-13 and coagulopathy, and prognosis in disseminated intravascular coagulation. Thromb Res. 2009; 124:75–79. PMID: 19162307.50. Ono T, Mimuro J, Madoiwa S, Soejima K, Kashiwakura Y, Ishiwata A, et al. Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006; 107:528–534. PMID: 16189276.51. Alhamdi Y, Abrams ST, Lane S, Wang G, Toh CH. Histone-associated thrombocytopenia in patients who are critically ill. JAMA. 2016; 315:817–819. PMID: 26903341.52. Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012; 122:2661–2671. PMID: 22684106.53. Goel R, Ness PM, Takemoto CM, Krishnamurti L, King KE, Tobian AA. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood. 2015; 125:1470–1476. PMID: 25588677.54. Yu M, Nardella A, Pechet L. Screening tests of disseminated intravascular coagulation: guidelines for rapid and specific laboratory diagnosis. Crit Care Med. 2000; 28:1777–1780. PMID: 10890618.55. Mackie IJ, Kitchen S, Machin SJ, Lowe GD. Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Guidelines on fibrinogen assays. Br J Haematol. 2003; 121:396–404. PMID: 12716361.56. Toh JM, Ken-Dror G, Downey C, Abrams ST. The clinical utility of fibrin-related biomarkers in sepsis. Blood Coagul Fibrinolysis. 2013; 24:839–843. PMID: 24030119.57. Nieuwenhuizen W. A reference material for harmonisation of D-dimer assays. Fibrinogen Subcommittee of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis. Thromb Haemost. 1997; 77:1031–1033. PMID: 9184423.58. Dempfle CE, Wurst M, Smolinski M, Lorenz S, Osika A, Olenik D, et al. Use of soluble fibrin antigen instead of D-dimer as fibrin-related marker may enhance the prognostic power of the ISTH overt DIC score. Thromb Haemost. 2004; 91:812–818. PMID: 15045145.59. Olson JD. D-dimer: An overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015; 69:1–46. PMID: 25934358.60. Choi Q, Hong KH, Kim JE, Kim HK. Changes in plasma levels of natural anticoagulants in disseminated intravascular coagulation: high prognostic value of antithrombin and protein C in patients with underlying sepsis or severe infection. Ann Lab Med. 2014; 34:85–91. PMID: 24624342.61. Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care. 2014; 18:R13. PMID: 24410881.62. Gando S, Kameue T, Nanzaki S, Nakanishi Y. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome. Thromb Haemost. 1996; 75:224–228. PMID: 8815564.63. Dempfle CE, Borggrefe M. Point of care coagulation tests in critically ill patients. Semin Thromb Hemost. 2008; 34:445–450. PMID: 18956284.64. Sivula M, Pettilä V, Niemi TT, Varpula M, Kuitunen AH. Thromboelastometry in patients with severe sepsis and disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2009; 20:419–426. PMID: 19581801.65. Haase N, Ostrowski SR, Wetterslev J, Lange T, Møller MH, Tousi H, et al. Thromboelastography in patients with severe sepsis: a prospective cohort study. Intensive Care Med. 2015; 41:77–85. PMID: 25413378.66. Müller MC, Meijers JC, Vroom MB, Juffermans NP. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care. 2014; 18:R30. PMID: 24512650.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Current Pathological and Laboratory Considerations in the Diagnosis of Disseminated Intravascular Coagulation
- Spontaneous Subdural Hematoma Associated with Disseminated Intravascular Coagulation in Patient with Cancer
- Disseminated intravascular coagulation
- Diagnostic Efficacy of Rapid Whole-Blood Assay for D-dimer in Patients with Disseminated Intravascular Coagulation
- Heparin Therapy for Disseminated Intravascular Coagulation in Childhood